Belgian health authorities announced Thursday they have inspected a pharmaceutical factory in Belgium to find out whether expected delays in the deliveries of AstraZeneca’s coronavirus vaccine are due to production issues.
The European Commission had asked the Belgian government to inspect the factory amid a heated public dispute between the 27-nation bloc and the Anglo-Swedish drugmaker. EU officials are under tremendous political pressures because the bloc's vaccine rollout has been much slower than that of Israel or Britain.
The Novasep’s factory in the town of Seneffe is part of the European production chain for the Oxford-AstraZeneca coronavirus vaccine.

European Commissioner in charge of Health Stella Kyriakides speaks during an online press conference on AstraZeneca at European Commission headquarters in Brussels, Wednesday, Jan. 27, 2021. (Olivier HosletPool Photo via AP)
AstraZeneca said last week that it planned to cut initial deliveries in the EU to 31 million doses from the 80 million it had planned due to reduced yields from its manufacturing plants in Europe. The EU claimed Wednesday that it will receive even less than that — just one quarter of the doses that member nations were supposed to get during January-March 2021.
According to the EU, the Belgian factory is one of four AstraZeneca sites included in the contract sealed by the European Commission and the company to produce vaccines for the EU market.
“The Novasep teams worked hard to meet its obligations to AstraZeneca with unprecedented speed and commitment," Novasep said in a statement to The Associated Press. “Manufacturing the COVID-19 vaccine is a pioneering process in terms of scale, complexity and quantity. We have worked closely with AstraZeneca and conducted regular and coordinated reviews of the production processes to ensure the active drug substance was delivered on time and met the highest standards for quality and stability."

Pedestrians, wearing face masks to prevent the spread of the coronavirus COVID-19, walk past the EU headquarters in Brussels, Wednesday, Jan. 27, 2021. The European Union and drugmaker AstraZeneca sparred Wednesday over a delay in coronavirus vaccine deliveries as the deepening dispute raises concerns about the increasing competition for limited supplies of shots needed to vaccinate populations and end the global pandemic. (AP PhotoFrancisco Seco)
France Dammel, a spokesperson for Belgium's health minister, said experts from the federal medicine agency inspected the Novasep site. They will now work with Dutch, Italian and Spanish experts before delivering a report in the coming days.
The EU said it expects to deliver the full amount on time and has threatened to put export inspections on all vaccines made in its territory. Stella Kyriakides, the European Commissioner for health and food safety, said AstraZeneca should provide vaccines from its U.K. facilities if it it is unable to meet commitments from factories in the EU.
After a third round of talks with AstraZeneca aimed at resolving the dispute on Wednesday evening, Kyriakides regretted the “continued lack of clarity on the delivery schedule” and urged AstraZeneca to come up with a clear plan for a quick delivery of the doses reserved by the EU for the first quarter.
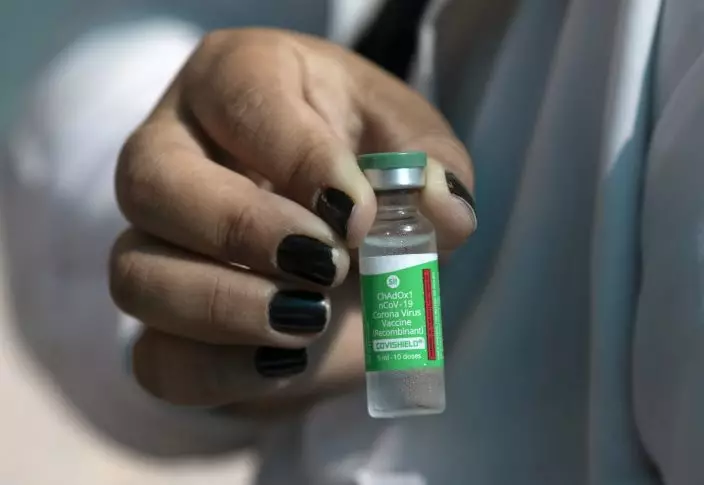
A healthcare worker shows a vial of the Oxford-AstraZeneca vaccine for COVID-19 under a tent where people are getting vaccinated at the "Clinica da Familia Estacio de Sa" in Rio de Janeiro, Brazil, Wednesday, Jan. 27, 2021. (AP PhotoSilvia Izquierdo)
A spokesman for AstraZeneca said after the meeting that the company has “committed to even closer coordination to jointly chart a path for the delivery of our vaccine over the coming months as we continue our efforts to bring this vaccine to millions of Europeans at no profit during the pandemic.”
The EU, which has 450 million people, has signed deals for six different vaccines, but so far regulators have only authorized the use of two, one made by Pfizer and another by Moderna. The EU’s drug regulator will consider the AstraZeneca vaccine on Friday.
Sylvain Plazy contributed to this story.
Follow all of AP’s pandemic coverage at:
https://apnews.com/hub/coronavirus-pandemic
https://apnews.com/hub/coronavirus-vaccine
https://apnews.com/UnderstandingtheOutbreak


