Improving indoor air quality in small spaces
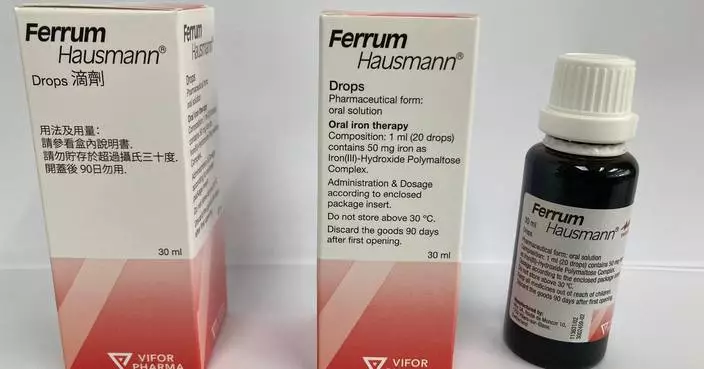
âThe Sham Shui Po District Office is facilitating donations of locally developed energy-efficient air purifiers, via community living rooms, to families in subdivided units, where PM2.5 concentrations are higher due to poor ventilation. The award-winning air purifier was developed by Lingnan University to specifically address the needs of subdivided households.
News.gov.hk spoke with District Officer (Sham Shui Po), Mr Paul Wong, to learn about how the Government utilised data to target households in need of the purifiers. A representative from Lingnan University and a recipient of one of the devices also spoke about the technology involved and its effectiveness.
The story is available at www.news.gov.hk/eng/feature/ today (May 26) in text and video format.

Source: AI-generated images
Pharmacy and poisons board of hong kong endorses regulation of medical gases as pharmaceutical products under ordinance, effective june 14, 2026
The Department of Health (DH) today (June 17) said that the Pharmacy and Poisons Board of Hong Kong (the Board) has endorsed the regulation of medical gases as pharmaceutical products under the Pharmacy and Poisons Ordinance (Cap. 138) (the Ordinance) with effect from June 14, 2026.
In September 2023, the Board agreed that medical gases should be regulated as pharmaceutical products under the Ordinance after taking into account the regulatory control of medical gases in other jurisdictions and the current situation in Hong Kong. In this connection, a public consultation had been conducted by the DH in November last year for collecting views and comments from the public and relevant stakeholders.
Noting that the overall responses were in support of the regulatory control of medical gases, the Board has decided at its meeting on June 14, 2024, to regulate medical gases as pharmaceutical products under the Ordinance by giving two years' preparatory time (i.e. from June 14, 2024, to June 13, 2026) for the trade to apply for relevant licences and registration of their products. The Board has also promulgated relevant Guidance Notes and details are available at the Board's website (www.ppbhk.org.hk/eng/index.html).
The DH has started issuing letters to inform relevant traders and stakeholders about the aforesaid regulation. When the new regulatory control takes effect, the medical gases, as pharmaceutical products, have to be registered with the Board before they can be legally sold or supplied in Hong Kong. In addition, traders of the pharmaceutical products must obtain relevant licence(s) from the Board before conducting manufacture, wholesale (including import and export) of pharmaceutical products and retail sales of pharmaceutical products containing poisons. According to the Ordinance, illegal possession or sale of unregistered pharmaceutical products or prescription drugs, and manufacture, wholesale of pharmaceutical products and retail sales of pharmaceutical products containing poisons without relevant licences are criminal offences. The maximum penalty for each offence is a fine of $100,000 and two years' imprisonment upon conviction.
For relevant information on the Regulation of Medical Gases, please visit the website of the Drug Office of the DH (www.drugoffice.gov.hk/eps/do/en/pharmaceutical_trade/medical_gases_regulation.html).
Source: AI-generated images