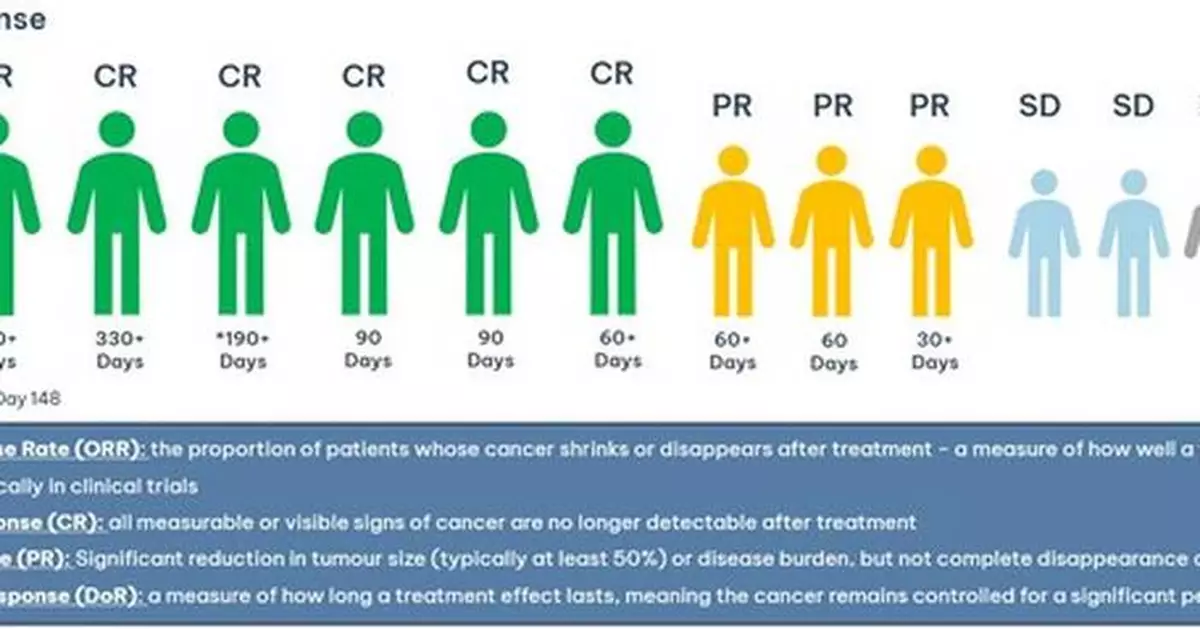
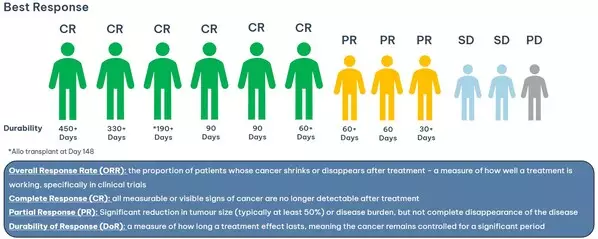
- Since the February update, an additional 5 patients have been dosed, resulting in 2 Complete Responses (CR) and 3 Partial Responses (PR)
- 75% Overall Response rate (ORR): 6 total CR and 3 PR in Phase 1b trial of azer-cel, an allogeneic off-the-shelf CD19 CAR T therapy in relapsed diffuse large B-cell lymphoma (DLBCL), an aggressive type of blood cancer
- First patient remains cancer free at 15 months and ongoing with additional patients having durable responses at 2, 5, and 11 months+ and durability data continuing to mature
- Patients in the trial have previously failed at least 3 lines of therapy with many patients failing 4-6 lines of therapy, including autologous CAR-T, reinforcing the potential of azer-cel in this high-unmet-need population
- Based on these positive results, Imugene expects to meet with the US FDA in Q4 2025 regarding a pivotal / registrational study for azer-cel
- Trial now open to enrol into CAR T naïve niche indications in other lymphomas
- Additional update expected in coming months
SYDNEY, July 14, 2025 /PRNewswire/ -- Imugene Limited (ASX: IMU), a clinical-stage immuno-oncology company, is pleased to announce exciting new data from its Phase 1b clinical trial evaluating azer-cel (azercabtagene zapreleucel) in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
In February 2025, Imugene announced that a total of four out of seven patients had achieved a Complete Response (CR), defined as the disappearance of all signs of cancer in response to treatment. Since then, two additional patients have also achieved a Complete Response, and three patients have achieved Partial Response (cancer reduction by at least 50%) bringing the best overall response rate to 75% and the CR rate to 55%. The duration of response continues to mature. These patients are being treated with azer-cel and interleukin 2 (IL -2).
| Evaluable | Treatment | N | Overall Response Rate (ORR) | Complete Response (CR) At Day 60 | Best Durability (Time of response) |
| DLBCL | Lymphodepletion (LD)1 +azer-cel +Interleukin-2 (IL-2) | 12 | 9/12 (75%) | 6/11 (55%) | >450 days on going |
For approved, autologous CD19 CART products, the average time to best response is 2-3 months with some patients taking up to 6 months to achieve their best response.
Azer-cel is being developed as a potential allogeneic, off-the-shelf, CAR T-cell therapy, addressing key limitations of approved autologous CAR T drugs, including geographical access to treatment centres, manufacturing complexity and time to receive treatment (on-demand).
Based on the updated response rate and maturing durability data, as well as having been awarded FDA Fast Track Designation for DLBCL in March 2025, Imugene will request a Type B (End of Phase 1) Meeting in Q4 2025, with the US FDA to present the data and to discuss designs for a pivotal / registrational trial for azer-cel.
Leslie Chong, Managing Director and CEO of Imugene, said:
"We are very pleased with the continued positive data coming from the azer-cel trial, which further reinforces its potential as a treatment for DLBCL patients who have failed several previous lines of therapy. The data also significantly improves our position from both a regulatory and commercial standpoint, and we look forward to expanding on these discussions with the FDA.
Additionally, given the positive results, we are opening the trial to other niche blood cancer indications, such as PCNSL and other subtypes of B Cell Lymphoma, for CAR T naïve patients. This is a high unmet need with potential to expedite and expand the scope of azer-cel."
Dr John Byon, Chief Medical Officer of Imugene, said:
"DLBCL remains one of the most aggressive forms of lymphoma, and despite the existing therapies, there are a large number of patients that still face relapse or resistance. We are seeing significant potential from azer-cel to date in its ability to provide a critical step forward for these patients who have relapsed on multiple therapies, offering deep and durable responses with a one-time treatment.
We remain deeply committed to transforming the standard of care in difficult-to-treat blood cancers, where significant unmet medical need still exists."
The FDA Fast Track Designation for DLBCL received for azer-cel is designed to facilitate the development and expedite the review of drugs that address serious or life-threatening conditions and meet an unmet medical need. Benefits of the designation include more frequent meetings with the FDA to discuss development plans, the option for rolling review of regulatory submissions, and potential eligibility for Accelerated Approval and Priority Review upon meeting relevant criteria.
Imugene continues to actively enrol patients into the Phase 1b azer-cel trial at ten US sites with up to six sites in Australia planned, after the first Australian patient was dosed in January 2025 at Royal Prince Alfred Hospital in Sydney, resulting in a Complete Response.
About the Phase 1b azer-cel trial
The azer-cel allogeneic CAR T trial is an ongoing, open-label, multi-centre Phase 1b clinical trial in the U.S. and Australia, for CAR T relapsed patients with DLBCL. The study has recently expanded to include and treat CAR T naïve patients diagnosed with a broad range of Non-Hodgkins lymphomas including primary central nervous system lymphoma (PCNSL), chronic lymphocytic leukemia (CLL)/ small lymphocytic lymphoma (SLL), marginal zone lymphoma (MZL), Waldenstrom macroglobulinemia (WM) and follicular lymphoma (FL). Treatment with azer-cel, lymphodepletion (LD) and IL-2 is showing promising results with evidence of meaningful clinical activity, and durability of response. Additionally, the safety profile is manageable and generally well tolerated.
About diffuse large B cell lymphoma (DLBCL)
DLBCL is an aggressive and fast-growing type of non-Hodgkin's lymphoma (NHL), a type of blood cancer. DLBCL is the most common type of NHL, with approximately 160,000[1] global cases per year and approximately 30,000 new cases per year in the U.S. Relapsed/refractory DLBCL has a high unmet medical need; ~60% of patients treated with approved autologous CD19 CAR T relapse.
| [1]Science Direct Volume 60, Issue 5, November 2023 |
About primary central nervous system lymphoma (PCNSL)
PCNSL is a rare and aggressive form of non-Hodgkin lymphoma (NHL), a type of blood cancer that originates in the brain, spinal cord, leptomeninges, or eyes, usually without evidence of systemic disease. In the U.S., there are approximately 1,500 to 1,800 new cases per year with limited approved treatment options and is a high unmet need. Currently, there are no CAR T-cell products approved for the treatment of PCNSL providing a unique opportunity for azer-cel to treat CART naïve patients.
About other types of B Cell Lymphoma
Other subtypes of non-Hodgkin lymphoma (NHL) include chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), the most common slow growing leukemia that can become resistant to therapy; marginal zone lymphoma (MZL), a slow-growing B-cell lymphoma that arises in lymphoid tissues associated with mucosal sites like the stomach and lung; Waldenström macroglobulinemia (WM), a rare slow-growing lymphoma characterized by excess IgM production, which can cause multiple complications ; and follicular lymphoma (FL), a common slow-growing NHL that can become more aggressive. While several targeted therapies and monoclonal antibodies are available for these types of B Cell Lymphoma, relapsed or refractory disease remains an ongoing challenge, highlighting the ongoing need for continued innovation and new and better treatments.
About Interleukin 2 (IL-2)
IL-2 is a cytokine (a protein that affects what happens between cells in the immune system) that helps T-cells (which are part of the immune system that help fight cancer) grow and survive. IL-2 has been shown to help T cells live longer and to enhance the cancer killing functions of CAR T cells, making them more effective at targeting and killing cancer cells.
For more information please contact:
Leslie Chong
Managing Director and Chief Executive Officer
info@imugene.com
General Investor Enquiries
shareholderenquiries@imugene.com
Media Enquiries
Matt Wright
matt@nwrcommunications.com.au
Connect with us on LinkedIn @Imugene Limited
Follow us on Twitter @TeamImugene
Watch us on YouTube @ImugeneLimited
About Imugene (ASX:IMU)
Imugene is a clinical stage immuno-oncology company developing a range of new and novel immunotherapies that seek to activate the immune system of cancer patients to treat and eradicate tumours. Our unique platform technologies seek to harness the body's immune system against tumours, potentially achieving a similar or greater effect than synthetically manufactured monoclonal antibody and other immunotherapies.
Our pipeline includes an off-the-shelf (allogeneic) cell therapy CAR T drug azer-cel (azercabtagene zapreleucel) which targets CD19 to treat blood cancers. Our pipeline also includes oncolytic virotherapy (CF33) aimed at treating a variety of cancers in combination with standard of care drugs and emerging immunotherapies such as CAR T's for solid tumours and B-cell vaccine candidates. We are supported by a leading team of international cancer experts with extensive experience in developing novel cancer therapies that are currently marketed globally.
Our vision is to help transform and improve the treatment of cancer and the lives of the millions of patients who need effective treatments. This vision is backed by a growing body of clinical evidence and peer-reviewed research. Together with leading specialists and medical professionals, we believe Imugene's immuno-oncology therapies will become foundation treatments for cancer. Our goal is to ensure that Imugene and its shareholders are at the forefront of this rapidly growing global market.
Release authorised by the Managing Director and Chief Executive Officer Imugene Limited.
SYDNEY, July 14, 2025 /PRNewswire/ -- Imugene Limited (ASX: IMU), a clinical-stage immuno-oncology company, is pleased to announce exciting new data from its Phase 1b clinical trial evaluating azer-cel (azercabtagene zapreleucel) in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
In February 2025, Imugene announced that a total of four out of seven patients had achieved a Complete Response (CR), defined as the disappearance of all signs of cancer in response to treatment. Since then, two additional patients have also achieved a Complete Response, and three patients have achieved Partial Response (cancer reduction by at least 50%) bringing the best overall response rate to 75% and the CR rate to 55%. The duration of response continues to mature. These patients are being treated with azer-cel and interleukin 2 (IL -2).
| Evaluable | Treatment | N | Overall Response Rate (ORR) | Complete Response (CR) At Day 60 | Best Durability (Time of response) |
| DLBCL | Lymphodepletion (LD)1 +azer-cel +Interleukin-2 (IL-2) | 12 | 9/12 (75%) | 6/11 (55%) | >450 days on going |
Evaluable
patients
Treatment
N
Overall Response Rate (ORR)
Complete Response (CR)
At Day 60
Best Durability (Time of response)
DLBCL
Lymphodepletion (LD)1 +azer-cel
+Interleukin-2 (IL-2)
12
9/12 (75%)
6/11 (55%)
>450 days on going
For approved, autologous CD19 CART products, the average time to best response is 2-3 months with some patients taking up to 6 months to achieve their best response.
Azer-cel is being developed as a potential allogeneic, off-the-shelf, CAR T-cell therapy, addressing key limitations of approved autologous CAR T drugs, including geographical access to treatment centres, manufacturing complexity and time to receive treatment (on-demand).
Based on the updated response rate and maturing durability data, as well as having been awarded FDA Fast Track Designation for DLBCL in March 2025, Imugene will request a Type B (End of Phase 1) Meeting in Q4 2025, with the US FDA to present the data and to discuss designs for a pivotal / registrational trial for azer-cel.
Leslie Chong, Managing Director and CEO of Imugene, said:
"We are very pleased with the continued positive data coming from the azer-cel trial, which further reinforces its potential as a treatment for DLBCL patients who have failed several previous lines of therapy. The data also significantly improves our position from both a regulatory and commercial standpoint, and we look forward to expanding on these discussions with the FDA.
Additionally, given the positive results, we are opening the trial to other niche blood cancer indications, such as PCNSL and other subtypes of B Cell Lymphoma, for CAR T naïve patients. This is a high unmet need with potential to expedite and expand the scope of azer-cel."
Dr John Byon, Chief Medical Officer of Imugene, said:
"DLBCL remains one of the most aggressive forms of lymphoma, and despite the existing therapies, there are a large number of patients that still face relapse or resistance. We are seeing significant potential from azer-cel to date in its ability to provide a critical step forward for these patients who have relapsed on multiple therapies, offering deep and durable responses with a one-time treatment.
We remain deeply committed to transforming the standard of care in difficult-to-treat blood cancers, where significant unmet medical need still exists."
The FDA Fast Track Designation for DLBCL received for azer-cel is designed to facilitate the development and expedite the review of drugs that address serious or life-threatening conditions and meet an unmet medical need. Benefits of the designation include more frequent meetings with the FDA to discuss development plans, the option for rolling review of regulatory submissions, and potential eligibility for Accelerated Approval and Priority Review upon meeting relevant criteria.
Imugene continues to actively enrol patients into the Phase 1b azer-cel trial at ten US sites with up to six sites in Australia planned, after the first Australian patient was dosed in January 2025 at Royal Prince Alfred Hospital in Sydney, resulting in a Complete Response.
About the Phase 1b azer-cel trial
The azer-cel allogeneic CAR T trial is an ongoing, open-label, multi-centre Phase 1b clinical trial in the U.S. and Australia, for CAR T relapsed patients with DLBCL. The study has recently expanded to include and treat CAR T naïve patients diagnosed with a broad range of Non-Hodgkins lymphomas including primary central nervous system lymphoma (PCNSL), chronic lymphocytic leukemia (CLL)/ small lymphocytic lymphoma (SLL), marginal zone lymphoma (MZL), Waldenstrom macroglobulinemia (WM) and follicular lymphoma (FL). Treatment with azer-cel, lymphodepletion (LD) and IL-2 is showing promising results with evidence of meaningful clinical activity, and durability of response. Additionally, the safety profile is manageable and generally well tolerated.
About diffuse large B cell lymphoma (DLBCL)
DLBCL is an aggressive and fast-growing type of non-Hodgkin's lymphoma (NHL), a type of blood cancer. DLBCL is the most common type of NHL, with approximately 160,000[1] global cases per year and approximately 30,000 new cases per year in the U.S. Relapsed/refractory DLBCL has a high unmet medical need; ~60% of patients treated with approved autologous CD19 CAR T relapse.
| [1]Science Direct Volume 60, Issue 5, November 2023 |
[1]Science Direct Volume 60, Issue 5, November 2023
About primary central nervous system lymphoma (PCNSL)
PCNSL is a rare and aggressive form of non-Hodgkin lymphoma (NHL), a type of blood cancer that originates in the brain, spinal cord, leptomeninges, or eyes, usually without evidence of systemic disease. In the U.S., there are approximately 1,500 to 1,800 new cases per year with limited approved treatment options and is a high unmet need. Currently, there are no CAR T-cell products approved for the treatment of PCNSL providing a unique opportunity for azer-cel to treat CART naïve patients.
About other types of B Cell Lymphoma
Other subtypes of non-Hodgkin lymphoma (NHL) include chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), the most common slow growing leukemia that can become resistant to therapy; marginal zone lymphoma (MZL), a slow-growing B-cell lymphoma that arises in lymphoid tissues associated with mucosal sites like the stomach and lung; Waldenström macroglobulinemia (WM), a rare slow-growing lymphoma characterized by excess IgM production, which can cause multiple complications ; and follicular lymphoma (FL), a common slow-growing NHL that can become more aggressive. While several targeted therapies and monoclonal antibodies are available for these types of B Cell Lymphoma, relapsed or refractory disease remains an ongoing challenge, highlighting the ongoing need for continued innovation and new and better treatments.
About Interleukin 2 (IL-2)
IL-2 is a cytokine (a protein that affects what happens between cells in the immune system) that helps T-cells (which are part of the immune system that help fight cancer) grow and survive. IL-2 has been shown to help T cells live longer and to enhance the cancer killing functions of CAR T cells, making them more effective at targeting and killing cancer cells.
For more information please contact:
Leslie Chong
Managing Director and Chief Executive Officer
info@imugene.com
General Investor Enquiries
shareholderenquiries@imugene.com
Media Enquiries
Matt Wright
matt@nwrcommunications.com.au
Connect with us on LinkedIn @Imugene Limited
Follow us on Twitter @TeamImugene
Watch us on YouTube @ImugeneLimited
About Imugene (ASX:IMU)
Imugene is a clinical stage immuno-oncology company developing a range of new and novel immunotherapies that seek to activate the immune system of cancer patients to treat and eradicate tumours. Our unique platform technologies seek to harness the body's immune system against tumours, potentially achieving a similar or greater effect than synthetically manufactured monoclonal antibody and other immunotherapies.
Our pipeline includes an off-the-shelf (allogeneic) cell therapy CAR T drug azer-cel (azercabtagene zapreleucel) which targets CD19 to treat blood cancers. Our pipeline also includes oncolytic virotherapy (CF33) aimed at treating a variety of cancers in combination with standard of care drugs and emerging immunotherapies such as CAR T's for solid tumours and B-cell vaccine candidates. We are supported by a leading team of international cancer experts with extensive experience in developing novel cancer therapies that are currently marketed globally.
Our vision is to help transform and improve the treatment of cancer and the lives of the millions of patients who need effective treatments. This vision is backed by a growing body of clinical evidence and peer-reviewed research. Together with leading specialists and medical professionals, we believe Imugene's immuno-oncology therapies will become foundation treatments for cancer. Our goal is to ensure that Imugene and its shareholders are at the forefront of this rapidly growing global market.
Release authorised by the Managing Director and Chief Executive Officer Imugene Limited.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Imugene Announces Outstanding Response Rates from the Phase 1b Trial of the Azer-cel Allogeneic CAR T in 3L+ DLBCL