SHANGHAI, Oct. 13, 2025 /PRNewswire/ -- Smartee Denti-Technology today announced the global launch of the Smartee Sleep Aligners, a clear aligner device designed to help manage obstructive sleep apnoea hypopnoea syndrome (OSAHS) and primary snoring (PS). The new series includes two versions: Smartee SA, which focuses on the treatment of OSAHS and PS, and Smartee SA Plus. The latter can treat OSAHS while simultaneously addressing orthodontic issues. Together, they provide clinicians with an invisible alternative to conventional OSAHS treatment therapies, offering greater comfort and compliance, and improving sleep quality.
Addressing a global health challenge
OSAHS is one of the most prevalent sleep-related breathing disorders. Early signs often include habitual snoring caused by airway narrowing. As the condition progresses, patients may experience repeated breathing interruptions during sleep, which not only impair rest quality but are also linked to serious cardiovascular and metabolic risks.
The Smartee Sleep Aligners Series addresses this challenge through digitally customized clear aligners with a dual-splint locking system that advances the mandible while distributing occlusal forces to stabilize the jaw. This mandibular repositioning increases the volume of the velopharyngeal and oropharyngeal airways, improving airflow and alleviating OSAHS symptoms. Additionally, the appliance helps to reposition the mandible, which also leads to improved tongue base positioning, contributing to an expanded pharyngeal airway and reduced obstruction.
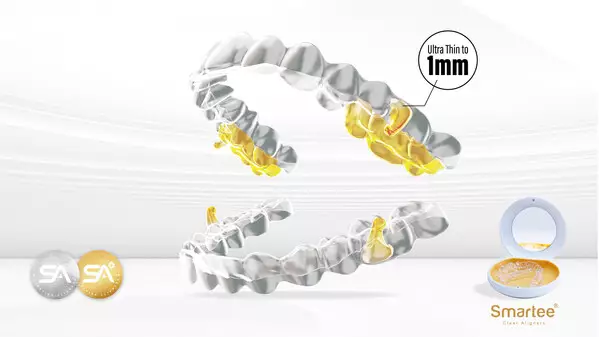
This device is made from a 1 mm-thick dental sheet, which offers enhanced comfort with minimal foreign body sensation. It demonstrates better patient acceptability and wear compliance.
There are two treatment options for OSAHS.
1. Smartee SA
- Indications: Simple snoring (without apnea), mild-to-moderate OSAHS, patients intolerant to CPAP, or those unsuitable for surgery.
- Function: Enlarge the velopharyngeal and oropharyngeal airway volume via mandibular advancement.
- Benefit: Titration assists clinicians in efficiently identifying the optimal mandibular advancement setting.
2. Smartee SA Plus
- Indications: Mild-to-moderate OSAHS combined with mild anterior misalignment.
- Function:
Daytime: Correcting anterior teeth with regular clear aligners.
Nighttime: Maintains airway patency and reduces snoring by wearing specially designed aligners with the mandibular advancement feature. - Benefit: Offers both integrated daytime orthodontic correction and nocturnal snore suppression.
Global Availability
Both appliances are now available for clinicians to order directly through the Smartee Case Management System. By combining digital orthodontics with airway management, the Smartee SA and SA Plus series underscores Smartee's commitment to delivering innovative, clinically proven, and patient-centric solutions for doctors and patients worldwide.
For more information about the Smartee Sleep Aligners, please visit: Smartee LinkedIn Newsletter
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Smartee Launches World's First Sleep Aligner Combining Anti-Snoring Therapy with Teeth Alignment










