|
– Aiming to establish af-001 as a new therapeutic option for radioactive iodine (RAI)-naïve patients with differentiated thyroid cancer –
TOKYO, Oct. 31, 2025 /PRNewswire/ -- Alpha Fusion Inc. (Headquarters: Chiyoda-ku, Tokyo; CEO: Sunao Fujioka; hereinafter "Alpha Fusion") today announced the initiation of a company-sponsored Phase I clinical trial (jRCT2031250472) of the alpha-emitting radiopharmaceutical af-001, which contains [211At]NaAt as its active pharmaceutical ingredient, in patients with differentiated thyroid cancer (papillary and follicular carcinoma).
This clinical trial is designed based on the results of an investigator-initiated Phase I study conducted at The University of Osaka, and consists of Part Ia and Part Ib.
In Part Ia, the maximum tolerated dose (MTD) of a single intravenous administration of af-001 will be determined in patients with differentiated thyroid cancer who are refractory to or intolerant of standard therapies. Part Ib will evaluate the efficacy and safety of multiple intravenous administrations of af-001 to determine the recommended dose for Phase II trials in patients with differentiated thyroid cancer who have not received prior radioactive iodine (RAI) therapy (RAI-naïve).
Part Ia of the study will be conducted at the National Cancer Center Hospital East (Principal Investigator: Dr. Makoto Tahara, Chief, Department of Head and Neck Medical Oncology), and Part Ib will be conducted at multiple sites in Japan.
The clinical development of af-001 will proceed with the goal of obtaining regulatory approval for use in patients with RAI-naïve differentiated thyroid cancer (papillary and follicular carcinoma). If clinical efficacy and safety are indicated in this patient population, af-001 is expected to contribute as a next-generation radiopharmaceutical and as a novel therapeutic option for differentiated thyroid cancer.
af-001 is manufactured under GMP for investigational products in collaboration with the Kobe City Medical Center General Hospital (Director: Dr. Yasuki Kihara; Chuo-ku, Kobe City, Hyogo), and promptly delivered to the National Cancer Center Hospital East after production.
(Reference: Prior release announced on PR Newswire, October 8, 2025)
Regarding the initiation of this study, Sunao Fujioka, CEO of Alpha Fusion Inc., commented as follows:
"Based on the achievements of the investigator-initiated Phase I study in patients refractory or intolerant to standard therapies, we expect to maximize the potential of af-001 by confirming its safety and efficacy in RAI-naïve patients. Alpha Fusion is fully committed to advancing the clinical development of af-001, with the ultimate goal of providing outpatient alpha-emitting radiopharmaceutical therapy to more patients with thyroid cancer. In addition, we are pursuing multiple research and development pipelines utilizing At-211, aiming to create innovative radiopharmaceuticals for various types of cancer."
Background
RAI therapy is the standard treatment for differentiated thyroid cancer. However, in cases involving multiple metastases or insufficient therapeutic response despite clear iodine uptake, repeated RAI treatments may be required.
Moreover, due to radiation protection requirements in Japan, RAI therapy must be administered in shielded rooms, leading to hospitalization that imposes psychological stress on patients and financial burdens on hospitals. A survey in Japan reported an average waiting time of approximately 3.7 months, and 23% of facilities having a wait time exceeding six months (Survey Report on the Utilization of RI Therapy Rooms for Thyroid Cancer, 2022).
Given these challenges, there is a growing demand for patient-centric radiopharmaceuticals that can achieve potent tumor-reducing effects in an outpatient setting.
About At-211 (af-001)
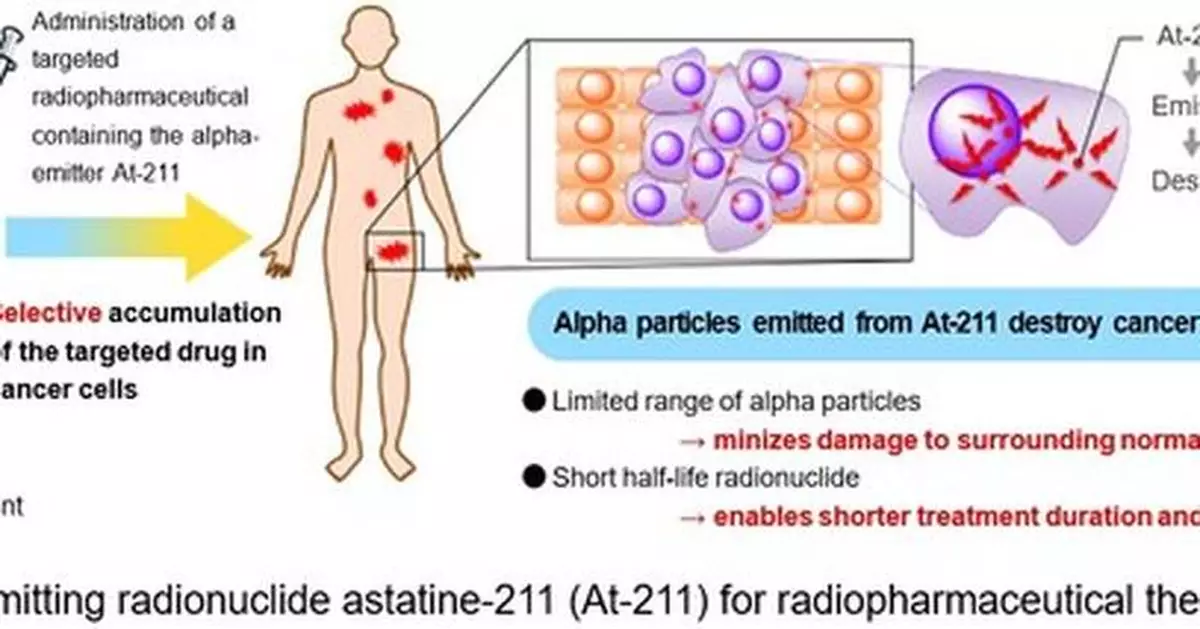
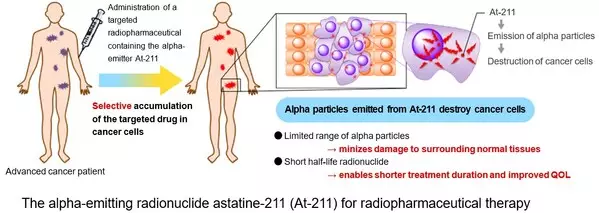
af-001 is a radiopharmaceutical containing astatine-211 (At-211) as its active pharmaceutical ingredient. It is selectively taken up by differentiated thyroid cancer cells through the sodium/iodide symporter (NIS) and exerts a cytotoxic effect by emitting alpha particles.
At-211 emits alpha particles with higher energy than beta particles, inducing double-strand DNA breaks in cancer cell nuclei and leading to tumor regression.
Because alpha particles have a very limited range (approximately several tens of micrometers), af-001 can deliver strong therapeutic effects while minimizing damage to surrounding normal tissues. Consequently, substituting RAI with the limited-range alpha emitter af-001 may enable outpatient-based treatment.
With these unique characteristics, af-001 is being developed as a next-generation radiopharmaceutical and a novel treatment for differentiated thyroid cancer, offering both efficacy and safety.
Results of the Investigator-Initiated Clinical Trial and Positioning of the Company-Sponsored Trial
Prior to this company-sponsored trial, an investigator-initiated Phase I clinical trial (Alpha-T1 Trial) of [211At]NaAt, identical in composition to af-001, was conducted at The University of Osaka under the supervision of Dr. Tadashi Watabe, Principal Investigator (NCT05275946).
The study indicated the safety and tolerability of [211At]NaAt in patients with differentiated thyroid cancer refractory or intolerant to standard therapies. Additionally, the study reported a ≥50% reduction in thyroglobulin concentration (a tumor marker) and cases of disappearance of 131I uptake on imaging.
(Source: Watabe T. First-in-Human Study of [211At]NaAt as Targeted Alpha Therapy in Patients with Radioiodine-Refractory Thyroid Cancer (Alpha-T1 Trial). J Nucl Med. September 25, 2025.)
The present company-sponsored trial aims to evaluate the efficacy and safety of af-001 (identical in pharmaceutical ingredient to TAH-1005, [211At]NaAt) in RAI-naïve patients, with the objective of determining the recommended dose for subsequent Phase II studies.
About Alpha Fusion Inc.
Alpha Fusion Inc. is a biopharmaceutical company dedicated to the research and development of next-generation radiopharmaceutical therapies utilizing radioactive isotopes.
Through collaborative partnerships with domestic and international medical and research institutions, Alpha Fusion aims to create new treatment options in oncology and contribute to the advancement of precision radiopharmaceutical medicine.
Official website: https://alpha-fusion.com/en/
– Aiming to establish af-001 as a new therapeutic option for radioactive iodine (RAI)-naïve patients with differentiated thyroid cancer –
TOKYO, Oct. 31, 2025 /PRNewswire/ -- Alpha Fusion Inc. (Headquarters: Chiyoda-ku, Tokyo; CEO: Sunao Fujioka; hereinafter "Alpha Fusion") today announced the initiation of a company-sponsored Phase I clinical trial (jRCT2031250472) of the alpha-emitting radiopharmaceutical af-001, which contains [211At]NaAt as its active pharmaceutical ingredient, in patients with differentiated thyroid cancer (papillary and follicular carcinoma).
This clinical trial is designed based on the results of an investigator-initiated Phase I study conducted at The University of Osaka, and consists of Part Ia and Part Ib.
In Part Ia, the maximum tolerated dose (MTD) of a single intravenous administration of af-001 will be determined in patients with differentiated thyroid cancer who are refractory to or intolerant of standard therapies. Part Ib will evaluate the efficacy and safety of multiple intravenous administrations of af-001 to determine the recommended dose for Phase II trials in patients with differentiated thyroid cancer who have not received prior radioactive iodine (RAI) therapy (RAI-naïve).
Part Ia of the study will be conducted at the National Cancer Center Hospital East (Principal Investigator: Dr. Makoto Tahara, Chief, Department of Head and Neck Medical Oncology), and Part Ib will be conducted at multiple sites in Japan.
The clinical development of af-001 will proceed with the goal of obtaining regulatory approval for use in patients with RAI-naïve differentiated thyroid cancer (papillary and follicular carcinoma). If clinical efficacy and safety are indicated in this patient population, af-001 is expected to contribute as a next-generation radiopharmaceutical and as a novel therapeutic option for differentiated thyroid cancer.
af-001 is manufactured under GMP for investigational products in collaboration with the Kobe City Medical Center General Hospital (Director: Dr. Yasuki Kihara; Chuo-ku, Kobe City, Hyogo), and promptly delivered to the National Cancer Center Hospital East after production.
(Reference: Prior release announced on PR Newswire, October 8, 2025)
Regarding the initiation of this study, Sunao Fujioka, CEO of Alpha Fusion Inc., commented as follows:
"Based on the achievements of the investigator-initiated Phase I study in patients refractory or intolerant to standard therapies, we expect to maximize the potential of af-001 by confirming its safety and efficacy in RAI-naïve patients. Alpha Fusion is fully committed to advancing the clinical development of af-001, with the ultimate goal of providing outpatient alpha-emitting radiopharmaceutical therapy to more patients with thyroid cancer. In addition, we are pursuing multiple research and development pipelines utilizing At-211, aiming to create innovative radiopharmaceuticals for various types of cancer."
Background
RAI therapy is the standard treatment for differentiated thyroid cancer. However, in cases involving multiple metastases or insufficient therapeutic response despite clear iodine uptake, repeated RAI treatments may be required.
Moreover, due to radiation protection requirements in Japan, RAI therapy must be administered in shielded rooms, leading to hospitalization that imposes psychological stress on patients and financial burdens on hospitals. A survey in Japan reported an average waiting time of approximately 3.7 months, and 23% of facilities having a wait time exceeding six months (Survey Report on the Utilization of RI Therapy Rooms for Thyroid Cancer, 2022).
Given these challenges, there is a growing demand for patient-centric radiopharmaceuticals that can achieve potent tumor-reducing effects in an outpatient setting.
About At-211 (af-001)
af-001 is a radiopharmaceutical containing astatine-211 (At-211) as its active pharmaceutical ingredient. It is selectively taken up by differentiated thyroid cancer cells through the sodium/iodide symporter (NIS) and exerts a cytotoxic effect by emitting alpha particles.
At-211 emits alpha particles with higher energy than beta particles, inducing double-strand DNA breaks in cancer cell nuclei and leading to tumor regression.
Because alpha particles have a very limited range (approximately several tens of micrometers), af-001 can deliver strong therapeutic effects while minimizing damage to surrounding normal tissues. Consequently, substituting RAI with the limited-range alpha emitter af-001 may enable outpatient-based treatment.
With these unique characteristics, af-001 is being developed as a next-generation radiopharmaceutical and a novel treatment for differentiated thyroid cancer, offering both efficacy and safety.
Results of the Investigator-Initiated Clinical Trial and Positioning of the Company-Sponsored Trial
Prior to this company-sponsored trial, an investigator-initiated Phase I clinical trial (Alpha-T1 Trial) of [211At]NaAt, identical in composition to af-001, was conducted at The University of Osaka under the supervision of Dr. Tadashi Watabe, Principal Investigator (NCT05275946).
The study indicated the safety and tolerability of [211At]NaAt in patients with differentiated thyroid cancer refractory or intolerant to standard therapies. Additionally, the study reported a ≥50% reduction in thyroglobulin concentration (a tumor marker) and cases of disappearance of 131I uptake on imaging.
(Source: Watabe T. First-in-Human Study of [211At]NaAt as Targeted Alpha Therapy in Patients with Radioiodine-Refractory Thyroid Cancer (Alpha-T1 Trial). J Nucl Med. September 25, 2025.)
The present company-sponsored trial aims to evaluate the efficacy and safety of af-001 (identical in pharmaceutical ingredient to TAH-1005, [211At]NaAt) in RAI-naïve patients, with the objective of determining the recommended dose for subsequent Phase II studies.
About Alpha Fusion Inc.
Alpha Fusion Inc. is a biopharmaceutical company dedicated to the research and development of next-generation radiopharmaceutical therapies utilizing radioactive isotopes.
Through collaborative partnerships with domestic and international medical and research institutions, Alpha Fusion aims to create new treatment options in oncology and contribute to the advancement of precision radiopharmaceutical medicine.
Official website: https://alpha-fusion.com/en/
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Alpha Fusion Initiates Company-Sponsored Clinical Trial of Alpha-Emitting Radiopharmaceutical af-001 in Patients with Differentiated Thyroid Cancer
ABU DHABI, UAE, Jan. 22, 2026 /PRNewswire/ -- The Department of Culture and Tourism – Abu Dhabi has appointed Elvira Dyangani Ose as Artistic Director of the Public Art Abu Dhabi Biennial to curate its second edition which opens in the autumn of 2026 and runs into 2027.
Building on the success of the inaugural edition that opened in 2024, the next edition of the Public Art Abu Dhabi Biennial, under Dyangani Ose's direction, will transform Abu Dhabi once again into an emirate-wide celebration of public art, culture and community.
The much-lauded inaugural edition of Public Art Abu Dhabi Biennial, which exhibited throughout Abu Dhabi and Al Ain, ran from 15 November 2024 to 30 April 2025. The biennale featured site-specific installations by artists from the UAE and around the world, many of which were then acquired to remain as permanent public works including pieces by Kader Attia, Superflex, Nathan Coley, Wael Al Awar, Farah Al Qassimi and Shaikha Al Ketbi.
Elvira Dyangani Ose currently serves as Director of the MACBA, Museu d'Art Contemporani de Barcelona. Previously, she was Director and Chief Curator at The Showroom in London. Among other initiatives, she has held the roles of Curator of the eighth Gothenburg International Biennial for Contemporary Art, Senior Curator at Creative Time, and Curator of International Art at Tate Modern. She sat on the Advisory Council of Tate Modern and was a member of the Thought Council of Fondazione Prada, for whom she curated numerous exhibitions. She currently sits on the recently appointed Board of CIMAM – International Committee for Museums and Collections of Modern Art.
Her curatorial projects, multidisciplinary in nature, reflect critically on the narration of history as a participatory experience, the traces of collective representation in public space, and the recovery of non-Western narratives and epistemologies. Her recent projects include Project a Black Planet – The Art and Culture of Panafrica (co-curated, 2024 – 2027), Coco Fusco. I Learned to Swim On Dry Land (2025) and Goshka Macuga's Miu Miu Tales and Tellers (convenor, 2024 - 2025).
Dyangani Ose holds a degree in Art History from the Universitat Autònoma de Barcelona. She is a doctoral candidate in Visual Studies at Cornell University in New York, where she earned a Master of Arts. She has a Diploma of Advanced Studies in Theory and History of Architecture from the Universitat Politècnica de Catalunya. She taught at Goldsmiths College as a specialist in Museology, Curating, Black Studies, and Contemporary African Art.
Further details and dates relating to the second edition of Public Art Abu Dhabi Biennial will be announced in due course.
Notes To Editors
About Public Art Abu Dhabi
Public Art Abu Dhabi is the Department of Culture and Tourism – Abu Dhabi (DCT Abu Dhabi)'s initiative under its ongoing commitment to commissioning public art for the emirate. Integral to this initiative is its community engagement efforts. The initiative advances the creative legacy, cultural infrastructure, liveability, and wellbeing of the UAE capital's residents through placemaking and collective memory. Public Art Abu Dhabi presents the Public Art Abu Dhabi Biennial along with Manar Abu Dhabi.
For more information please visit: paad.ae
About the Department of Culture and Tourism – Abu Dhabi:
The Department of Culture and Tourism – Abu Dhabi (DCT Abu Dhabi) drives the sustainable growth of Abu Dhabi's culture and tourism sectors and its creative industries, fuelling economic progress and helping to achieve Abu Dhabi's wider global ambitions.
By working in partnership with the organisations that define the emirate's position as a leading international destination, DCT Abu Dhabi strives to unite the ecosystem around a shared vision of the emirate's potential, coordinate effort and investment, deliver innovative solutions, and use the best tools, policies and systems to support the culture and tourism.
DCT Abu Dhabi's vision is defined by the emirate's people, heritage and landscape. We work to enhance Abu Dhabi's status as a place of authenticity, innovation, and unparalleled experiences, represented by its living traditions of hospitality, pioneering initiatives and creative thought.
For more information about the Department of Culture and Tourism – Abu Dhabi and the destination, please visit: dct.gov.ae and abudhabiculture.ae
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Elvira Dyangani Ose Appointed Artistic Director of the Second Edition of Public Art Abu Dhabi Biennial