|
BEIJING, Dec. 16, 2025 /PRNewswire/ -- METiS TechBio ("METiS") today announced that two of its oncology pipeline candidates, MTS-105 and MTS-107, have been published in leading international peer-reviewed journals, Nature Communications and the Journal for ImmunoTherapy of Cancer (JITC), representing two major breakthroughs in mRNA-based cancer therapeutics.
Both studies leverage METiS's proprietary AI-powered NanoForge platform, which introduces a precision-guided rocket-and-payload delivery analogy. By combining liver- and spleen-targeted lipid nanoparticle (LNP) delivery systems with programmable mRNA engineering, METiS has developed a new generation of immunotherapy strategies capable of efficiently activating antitumor immunity in specific organs in vivo.
MTS-105 is a first-in-class mRNA-encoded T cell engager (TCE) therapy candidate for hepatocellular carcinoma (HCC), delivered via METiS's proprietary liver-targeted LNP system. Study results demonstrate that, once delivered to and taken up within the liver, the mRNA is translated in situ and secreted locally as high level of bispecific antibodies, which rapidly penetrate HCC tissues. Employing this "Trojan Horse" strategy, MTS-105 efficiently activates T cells within the tumor to induce tumor cell killing, achieving complete tumor clearance and long-term T cell immune memory in mouse models.
MTS-105 provides a breakthrough solution to the long-standing limitations of protein-based TCEs in solid tumors, positioned as the world's first mRNA-encoded TCE therapy for solid tumors. The program has now entered clinical development.
MTS-107 is an innovative mRNA therapeutic vaccine targeting HPV16/18-positive cervical and head and neck cancers, with potential to achieve breakthrough treatment for HPV-associated cancers. Using spleen-targeted LNPs, METiS's team designed a construct combining dual E6/E7 antigens with a novel immune-activating adjuvant. In mouse models, MTS-107 demonstrated synergistic antitumor activity with PD-1 checkpoint inhibitors, resulting in robust expansion of HPV-specific CD8⁺T cells. MTS-107 will continue into original clinical exploration.
Chris LAI, Co-founder and CEO, said:
"This is an important milestone demonstrating the power of METiS's AI-driven NanoForge platform in therapeutic development. In conventional cancer therapy, most 'soldiers' remain outside the tumor, unable to infiltrate solid tumors for precise, effective killing. Our 'rocket-and-satellite' precision delivery paradigm has been strongly validated in these studies. We look forward to advancing global clinical development with our partners, bringing targeted therapies to patients and offering hope for survival or even cure."
Dr. Wei XU, Chief Scientific Officer and corresponding author of two studies, added:
"mRNA therapeutics have long been constrained by delivery challenges, and innovation in LNP technology is essential for unlocking their full potential. MTS-105 is the first to show that an Fc-free bispecific T cell engager can activate T cells without driving exhaustion, while MTS-107 introduces a new antigen design and built-in adjuvant that markedly enhance antitumor immunity.
A recent Nature study reported that patients treated with PD-1 inhibitors nearly doubled their three-year survival if they had also received an mRNA COVID-19 vaccine—a finding that strongly aligns with our observations for MTS-107. These results highlight how mRNA platforms can synergize with PD-1 blockade and signal a new chapter for immuno-oncology"
Dr. Andong LIU, Vice President and Head of Platform Technologies, and co-corresponding author of the Nature Communications study, stated:
"Our NanoForge engine significantly accelerates LNP and mRNA design cycles, boosting delivery efficiency and safety for liver- and spleen-targeted therapeutics. LNP nanodelivery is critical for precise tissue targeting and effective antitumor therapy. These studies open broad new avenues for innovative mRNA therapeutics."
MTS-105: Preclinical Breakthrough in Nature Communications
On December 15, Nature Communications (IF 15.7, 2024) published METiS's preclinical study titled:
"Organ-Specific Delivery of an mRNA-Encoded Bispecific T Cell Engager Targeting Glypican-3 in Hepatocellular Carcinoma."
Key highlights:
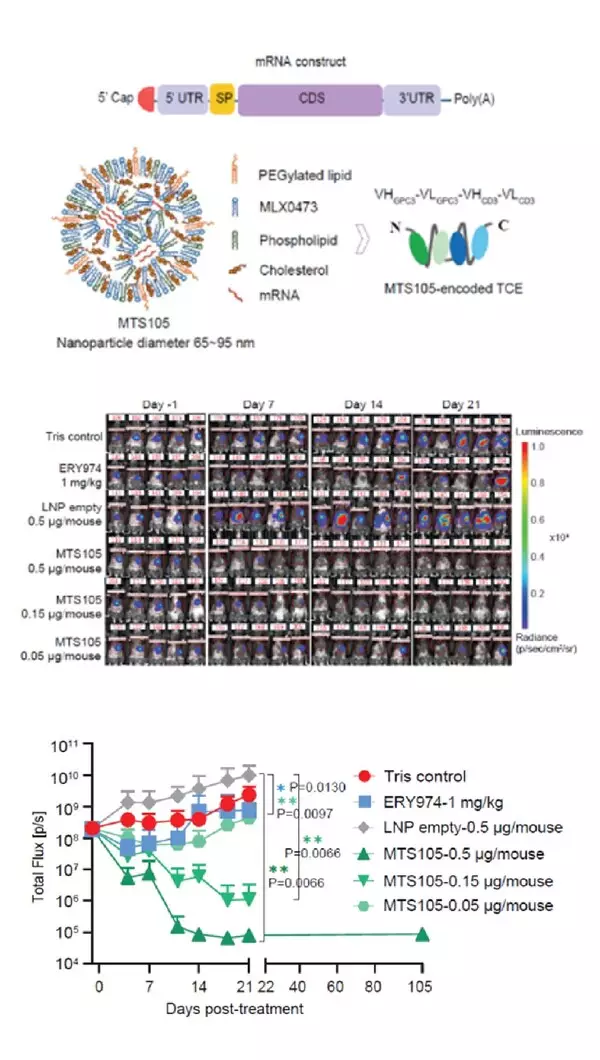
- Organ-Specific Delivery: MTS-105 achieves highly efficient liver-targeted delivery with minimal systemic exposure. Studies in mice, rats, and cynomolgus monkeys show superior hepatic enrichment compared to antibody-based TCEs.
- Controlled Release and Safety: Reduced C max and systemic exposure lower toxicity risks. In cynomolgus monkeys, linear pharmacokinetics were observed with excellent tolerability, supporting potential weekly dosing.
- Potent Antitumor Efficacy: Data from mouse models showed a marked increase in intratumoral exposure, enabling 100% complete responses—with full tumor clearance—at doses as low as 0.15 μg. In contrast, the protein-based TCE control achieved only ~50% tumor growth inhibition even at 1 mg/kg (approximately 20 μg).
- Durable T Cell Memory: Cured mice remained tumor-free upon rechallenge, indicating long-term immune memory and prevention of recurrence.
MTS-105 sets a new paradigm for translating TCE therapy into solid tumors, paving the way for first-in-class mRNA-encoded TCE therapy.
MTS-107: Breakthrough HPV Vaccine Published in JITC
On September 17, JITC published: "mRNA-encoded mutant HPV16/18 vaccines promote specific T-cell responses and synergize with anti-PD-1 checkpoint blockade in mediating therapeutic tumor regression in mice."
Key findings:
- Complete Tumor Regression: In advanced HPV18⁺MC38 tumor models, MTS-107 combined with PD-1 blockade achieved 100% complete response (CR).
- Spleen-Targeted LNP Delivery: Enhances antigen presentation and T cell activation.
- Dual-Subtype Antigen Design: Encodes mutated HPV16/18 E6/E7 antigens; optimized mRNA sequences improve translation and stability.
- Built-in Adjuvant: Co-expresses GM-CSF to promote dendritic cell maturation and HPV-specific CD8⁺T cell activation.
- Synergy with PD-1 Blockade: While monotherapy expands tumor-infiltrating HPV-specific T cells; combination with PD-1 blockade relieves immune suppression for complete tumor regression.
This work demonstrates the transformative potential of AI-powered mRNA vaccines in HPV-associated cancers and establishes METiS's innovation at the intersection of AI-driven nanodelivery and tumor immunotherapy, providing a strong foundation for clinical translation.
*Reference: Grippin, et al. Nature 2025, https://doi.org/10.1038/s41586-025-09655-y
BEIJING, Dec. 16, 2025 /PRNewswire/ -- METiS TechBio ("METiS") today announced that two of its oncology pipeline candidates, MTS-105 and MTS-107, have been published in leading international peer-reviewed journals, Nature Communications and the Journal for ImmunoTherapy of Cancer (JITC), representing two major breakthroughs in mRNA-based cancer therapeutics.
Both studies leverage METiS's proprietary AI-powered NanoForge platform, which introduces a precision-guided rocket-and-payload delivery analogy. By combining liver- and spleen-targeted lipid nanoparticle (LNP) delivery systems with programmable mRNA engineering, METiS has developed a new generation of immunotherapy strategies capable of efficiently activating antitumor immunity in specific organs in vivo.
MTS-105 is a first-in-class mRNA-encoded T cell engager (TCE) therapy candidate for hepatocellular carcinoma (HCC), delivered via METiS's proprietary liver-targeted LNP system. Study results demonstrate that, once delivered to and taken up within the liver, the mRNA is translated in situ and secreted locally as high level of bispecific antibodies, which rapidly penetrate HCC tissues. Employing this "Trojan Horse" strategy, MTS-105 efficiently activates T cells within the tumor to induce tumor cell killing, achieving complete tumor clearance and long-term T cell immune memory in mouse models.
MTS-105 provides a breakthrough solution to the long-standing limitations of protein-based TCEs in solid tumors, positioned as the world's first mRNA-encoded TCE therapy for solid tumors. The program has now entered clinical development.
MTS-107 is an innovative mRNA therapeutic vaccine targeting HPV16/18-positive cervical and head and neck cancers, with potential to achieve breakthrough treatment for HPV-associated cancers. Using spleen-targeted LNPs, METiS's team designed a construct combining dual E6/E7 antigens with a novel immune-activating adjuvant. In mouse models, MTS-107 demonstrated synergistic antitumor activity with PD-1 checkpoint inhibitors, resulting in robust expansion of HPV-specific CD8⁺T cells. MTS-107 will continue into original clinical exploration.
Chris LAI, Co-founder and CEO, said:
"This is an important milestone demonstrating the power of METiS's AI-driven NanoForge platform in therapeutic development. In conventional cancer therapy, most 'soldiers' remain outside the tumor, unable to infiltrate solid tumors for precise, effective killing. Our 'rocket-and-satellite' precision delivery paradigm has been strongly validated in these studies. We look forward to advancing global clinical development with our partners, bringing targeted therapies to patients and offering hope for survival or even cure."
Dr. Wei XU, Chief Scientific Officer and corresponding author of two studies, added:
"mRNA therapeutics have long been constrained by delivery challenges, and innovation in LNP technology is essential for unlocking their full potential. MTS-105 is the first to show that an Fc-free bispecific T cell engager can activate T cells without driving exhaustion, while MTS-107 introduces a new antigen design and built-in adjuvant that markedly enhance antitumor immunity.
A recent Nature study reported that patients treated with PD-1 inhibitors nearly doubled their three-year survival if they had also received an mRNA COVID-19 vaccine—a finding that strongly aligns with our observations for MTS-107. These results highlight how mRNA platforms can synergize with PD-1 blockade and signal a new chapter for immuno-oncology"
Dr. Andong LIU, Vice President and Head of Platform Technologies, and co-corresponding author of the Nature Communications study, stated:
"Our NanoForge engine significantly accelerates LNP and mRNA design cycles, boosting delivery efficiency and safety for liver- and spleen-targeted therapeutics. LNP nanodelivery is critical for precise tissue targeting and effective antitumor therapy. These studies open broad new avenues for innovative mRNA therapeutics."
MTS-105: Preclinical Breakthrough in Nature Communications
On December 15, Nature Communications (IF 15.7, 2024) published METiS's preclinical study titled:
"Organ-Specific Delivery of an mRNA-Encoded Bispecific T Cell Engager Targeting Glypican-3 in Hepatocellular Carcinoma."
Key highlights:
- Organ-Specific Delivery: MTS-105 achieves highly efficient liver-targeted delivery with minimal systemic exposure. Studies in mice, rats, and cynomolgus monkeys show superior hepatic enrichment compared to antibody-based TCEs.
- Controlled Release and Safety: Reduced C max and systemic exposure lower toxicity risks. In cynomolgus monkeys, linear pharmacokinetics were observed with excellent tolerability, supporting potential weekly dosing.
- Potent Antitumor Efficacy: Data from mouse models showed a marked increase in intratumoral exposure, enabling 100% complete responses—with full tumor clearance—at doses as low as 0.15 μg. In contrast, the protein-based TCE control achieved only ~50% tumor growth inhibition even at 1 mg/kg (approximately 20 μg).
- Durable T Cell Memory: Cured mice remained tumor-free upon rechallenge, indicating long-term immune memory and prevention of recurrence.
MTS-105 sets a new paradigm for translating TCE therapy into solid tumors, paving the way for first-in-class mRNA-encoded TCE therapy.
MTS-107: Breakthrough HPV Vaccine Published in JITC
On September 17, JITC published: "mRNA-encoded mutant HPV16/18 vaccines promote specific T-cell responses and synergize with anti-PD-1 checkpoint blockade in mediating therapeutic tumor regression in mice."
Key findings:
- Complete Tumor Regression: In advanced HPV18⁺MC38 tumor models, MTS-107 combined with PD-1 blockade achieved 100% complete response (CR).
- Spleen-Targeted LNP Delivery: Enhances antigen presentation and T cell activation.
- Dual-Subtype Antigen Design: Encodes mutated HPV16/18 E6/E7 antigens; optimized mRNA sequences improve translation and stability.
- Built-in Adjuvant: Co-expresses GM-CSF to promote dendritic cell maturation and HPV-specific CD8⁺T cell activation.
- Synergy with PD-1 Blockade: While monotherapy expands tumor-infiltrating HPV-specific T cells; combination with PD-1 blockade relieves immune suppression for complete tumor regression.
This work demonstrates the transformative potential of AI-powered mRNA vaccines in HPV-associated cancers and establishes METiS's innovation at the intersection of AI-driven nanodelivery and tumor immunotherapy, providing a strong foundation for clinical translation.
*Reference: Grippin, et al. Nature 2025, https://doi.org/10.1038/s41586-025-09655-y
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Breakthrough Progress: METiS TechBio Publishes Consecutive Research Findings in Nature Communications and the Journal for ImmunoTherapy of Cancer
Breakthrough Progress: METiS TechBio Publishes Consecutive Research Findings in Nature Communications and the Journal for ImmunoTherapy of Cancer
Breakthrough Progress: METiS TechBio Publishes Consecutive Research Findings in Nature Communications and the Journal for ImmunoTherapy of Cancer
|
DUBAI, UAE, Dec. 16, 2025 /PRNewswire/ -- Bybit, the world's second-largest cryptocurrency exchange by trading volume, has announced the launch of a new mETH Boosted Yield Campaign on Bybit On-Chain Earn in partnership with Mantle and mETH Protocol, offering a fixed 3 percent Bonus APR on eligible newly minted mETH during the event period in conjunction with the rollout of the mETH Buffer Pool Upgrade.
The limited-time campaign runs from Dec. 16, 2025, at 00:00 UTC through Feb. 15, 2026, at 23:59 UTC and marks a significant development in liquid staking offerings on the Bybit platform. Through the upgraded Buffer Pool architecture, mETH now targets redemption times of approximately 24 hours, positioning the asset as a faster, more flexible Ethereum-native yield instrument.
The initiative reflects Bybit's continued expansion of its On-Chain Earn suite and its strategic collaboration with mETH Protocol. Users can stake ETH to mint mETH directly through the Bybit platform without the need for external wallets, cross-chain transfers, or manual delegation processes.
During the event period, participants receive a fixed 3 percent Bonus APR on all new mETH minted via Bybit On Chain Earn (on top of the standard Staking APR). The campaign also introduces faster liquidity through the Buffer Pool mechanism, which is designed to significantly reduce redemption timelines compared with traditional Ethereum unstaking periods that can range from several days to weeks.
mETH is the receipt token issued for ETH staked via mETH Protocol, part of the Mantle Liquid Staking Program. It is a value-accruing asset designed to deliver one of the higher sustainable staking yields in the market. The newly upgraded Buffer Pool integrates a dynamic liquidity model supported by Aave, enabling near-immediate withdrawals under normal liquidity conditions.
Bybit users benefit from enhanced liquidity access, an exclusive yield subsidy during the campaign period, and full collateral utility. mETH is supported as a collateral asset within Bybit Unified Trading Accounts, allowing eligible users to maintain trading activity while earning staking rewards.
Eligibility calculations are based on hourly balance snapshots, with each user's daily eligible mETH amount determined by the lowest recorded balance within a 24-hour period. Participation includes Main Accounts and Subaccounts such as Funding, Trading Bot, and Unified Trading Accounts, while certain uses of mETH, including assets borrowed or pledged within Crypto Loans or held in Copy Trading Pro Subaccounts, are excluded. Bonus rewards are distributed daily to users' Funding Accounts.
The mETH Boosted Yield Campaign underscores Bybit's focus on improving capital efficiency and liquidity for Ethereum staking participants while expanding access to institutional-grade on-chain yield solutions through a centralized platform.
More information is available here.
#Bybit / #TheCryptoArk / #IMakeIt
About Bybit
Bybit is the world's second-largest cryptocurrency exchange by trading volume, serving a global community of over 70 million users. Founded in 2018, Bybit is redefining openness in the decentralized world by creating a simpler, open and equal ecosystem for everyone. With a strong focus on Web3, Bybit partners strategically with leading blockchain protocols to provide robust infrastructure and drive on-chain innovation. Renowned for its secure custody, diverse marketplaces, intuitive user experience, and advanced blockchain tools, Bybit bridges the gap between TradFi and DeFi, empowering builders, creators, and enthusiasts to unlock the full potential of Web3. Discover the future of decentralized finance at Bybit.com.
For more details about Bybit, please visit Bybit Press
For media inquiries, please contact: media@bybit.com
For updates, please follow: Bybit's Communities and Social Media
Discord | Facebook | Instagram | LinkedIn | Reddit | Telegram | TikTok | X | Youtube
DUBAI, UAE, Dec. 16, 2025 /PRNewswire/ -- Bybit, the world's second-largest cryptocurrency exchange by trading volume, has announced the launch of a new mETH Boosted Yield Campaign on Bybit On-Chain Earn in partnership with Mantle and mETH Protocol, offering a fixed 3 percent Bonus APR on eligible newly minted mETH during the event period in conjunction with the rollout of the mETH Buffer Pool Upgrade.
The limited-time campaign runs from Dec. 16, 2025, at 00:00 UTC through Feb. 15, 2026, at 23:59 UTC and marks a significant development in liquid staking offerings on the Bybit platform. Through the upgraded Buffer Pool architecture, mETH now targets redemption times of approximately 24 hours, positioning the asset as a faster, more flexible Ethereum-native yield instrument.
The initiative reflects Bybit's continued expansion of its On-Chain Earn suite and its strategic collaboration with mETH Protocol. Users can stake ETH to mint mETH directly through the Bybit platform without the need for external wallets, cross-chain transfers, or manual delegation processes.
During the event period, participants receive a fixed 3 percent Bonus APR on all new mETH minted via Bybit On Chain Earn (on top of the standard Staking APR). The campaign also introduces faster liquidity through the Buffer Pool mechanism, which is designed to significantly reduce redemption timelines compared with traditional Ethereum unstaking periods that can range from several days to weeks.
mETH is the receipt token issued for ETH staked via mETH Protocol, part of the Mantle Liquid Staking Program. It is a value-accruing asset designed to deliver one of the higher sustainable staking yields in the market. The newly upgraded Buffer Pool integrates a dynamic liquidity model supported by Aave, enabling near-immediate withdrawals under normal liquidity conditions.
Bybit users benefit from enhanced liquidity access, an exclusive yield subsidy during the campaign period, and full collateral utility. mETH is supported as a collateral asset within Bybit Unified Trading Accounts, allowing eligible users to maintain trading activity while earning staking rewards.
Eligibility calculations are based on hourly balance snapshots, with each user's daily eligible mETH amount determined by the lowest recorded balance within a 24-hour period. Participation includes Main Accounts and Subaccounts such as Funding, Trading Bot, and Unified Trading Accounts, while certain uses of mETH, including assets borrowed or pledged within Crypto Loans or held in Copy Trading Pro Subaccounts, are excluded. Bonus rewards are distributed daily to users' Funding Accounts.
The mETH Boosted Yield Campaign underscores Bybit's focus on improving capital efficiency and liquidity for Ethereum staking participants while expanding access to institutional-grade on-chain yield solutions through a centralized platform.
More information is available here.
#Bybit / #TheCryptoArk / #IMakeIt
About Bybit
Bybit is the world's second-largest cryptocurrency exchange by trading volume, serving a global community of over 70 million users. Founded in 2018, Bybit is redefining openness in the decentralized world by creating a simpler, open and equal ecosystem for everyone. With a strong focus on Web3, Bybit partners strategically with leading blockchain protocols to provide robust infrastructure and drive on-chain innovation. Renowned for its secure custody, diverse marketplaces, intuitive user experience, and advanced blockchain tools, Bybit bridges the gap between TradFi and DeFi, empowering builders, creators, and enthusiasts to unlock the full potential of Web3. Discover the future of decentralized finance at Bybit.com.
For more details about Bybit, please visit Bybit Press
For media inquiries, please contact: media@bybit.com
For updates, please follow: Bybit's Communities and Social Media
Discord | Facebook | Instagram | LinkedIn | Reddit | Telegram | TikTok | X | Youtube
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Bybit Launches mETH Boosted Yield Campaign With Fixed 3% Bonus APR on On-Chain Earn