|
- Following AQUA and SCALE PET, the latest FDA clearance reinforces Neurophet's leadership in AI-based Alzheimer's disease imaging analysis
- Advanced analysis of neuroimaging features associated with cerebral microbleeds and edema supports imaging-based clinical decision-making and accelerates U.S. market expansion
SEOUL, South Korea, Feb. 6, 2026 /PRNewswire/ -- Neurophet (Co-CEOs Jake Junkil Been and Donghyeon Kim), an artificial intelligence (AI) solution company for brain disorders diagnosis and treatment, announced today that its software solution, Neurophet AQUA AD Plus, a comprehensive neuroimaging analysis solution for clinical evaluation related to Alzheimer's disease, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
This clearance marks Neurophet's third FDA 510(k) clearance, following Neurophet AQUA, a brain neurodegeneration imaging analysis software, and Neurophet SCALE PET, a PET image quantitative analysis software. The achievement further validates the safety and effectiveness of Neurophet's core product portfolio at a global regulatory standard.
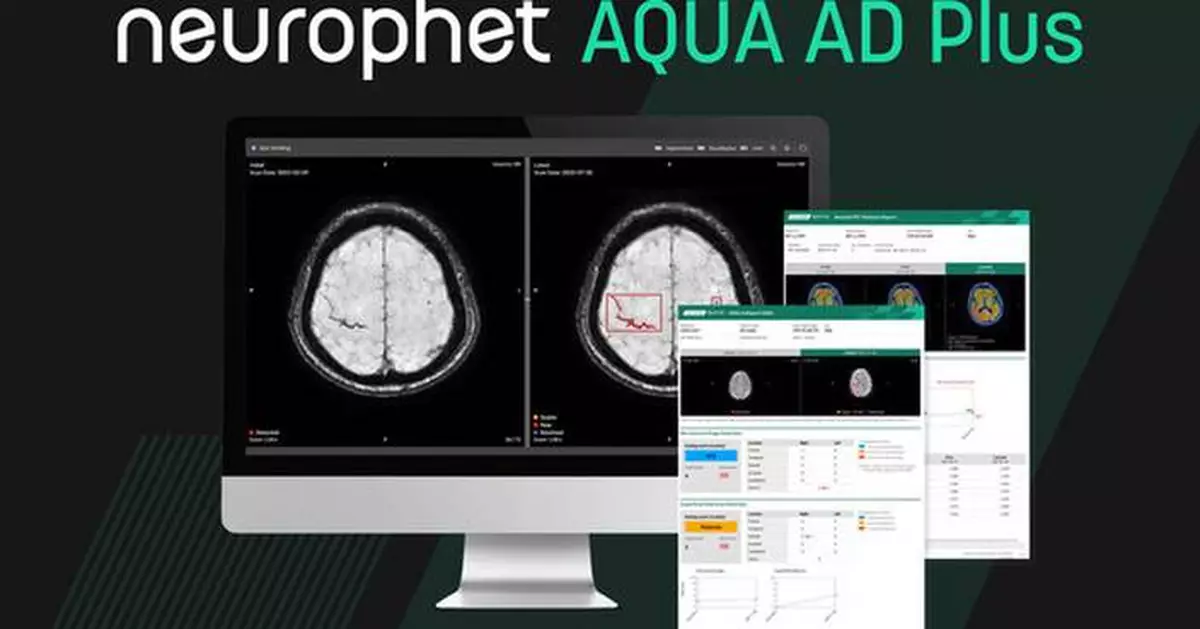
Neurophet AQUA AD Plus is a software-based solution designed to support imaging-based clinical evaluation across the Alzheimer's disease care continuum. The software performs quantitative analysis of MRI and PET images, enabling automated labeling, visualization, volumetric quantification of brain structures and lesions, as well as standardized uptake value ratio (SUVR) analysis. Quantitative results can be compared with normative reference data to support the evaluation of neurodegeneration and cognitive impairment.
The FDA-cleared U.S. version of Neurophet AQUA AD Plus is an upgraded solution that builds upon the capabilities of Neurophet AQUA AD. Leveraging AI-based brain MRI analysis, the software automatically analyzes and quantifies:
- Hypointense lesions associated with cerebral microbleeds and superficial siderosis, and
- Hyperintense lesions related to brain edema
By providing automated lesion localization and counts, the software helps clinicians more precise assess imaging-based risk factors and supports informed, patient-specific clinical decision-making when evaluating patients undergoing Alzheimer's disease-related therapeutic management.
"Receiving FDA 510(k) clearance for Neurophet AQUA AD Plus represents a significant milestone that allows us to introduce our advanced Alzheimer's imaging technology more broadly in the U.S. market," said Jake Junkil Been, Co-CEO of Neurophet. "We plan to accelerate our expansion across the U.S. by strengthening collaborations with healthcare institutions and strategic partners."
Neurophet AQUA AD Plus was previously designated as an Innovative Medical Technology in South Korea in September 2025. Building on this momentum, Neurophet plans to progressively expand clinical adoption of the solution across global healthcare settings, further broadening its global footprint in AI-based neuroimaging.
About Neurophet
Neurophet specializes in developing solutions for diagnosis support, treatment guides, and treatment devices targeting brain disorders based on cutting-edge artificial intelligence (AI) technology. The company was founded in 2016 by Co-CEOs Jake Junkil Been and Donghyeon Kim, who developed the next-generation neuro-navigation system.
Major products include brain MRI analysis software "Neurophet AQUA", PET Image Quantitative Analysis Software "Neurophet SCALE PET", Brain imaging treatment planning software for electric and magnetic brain stimulation "Neurophet tES/TMS LAB", Alzheimer's Disease treatment prescription and monitoring software "Neurophet AQUA AD" for tracking treatment efficacy and side effects, and Multiple Sclerosis image analysis software "Neurophet AQUA MS".
Neurophet has set its top priority to helping patients suffering from brain disorders. Based on expertise in neuroscience, Neurophet will continue to challenge and grow to explore the human brain's health and pioneer solutions for brain diseases with AI technology.
- Following AQUA and SCALE PET, the latest FDA clearance reinforces Neurophet's leadership in AI-based Alzheimer's disease imaging analysis
- Advanced analysis of neuroimaging features associated with cerebral microbleeds and edema supports imaging-based clinical decision-making and accelerates U.S. market expansion
SEOUL, South Korea, Feb. 6, 2026 /PRNewswire/ -- Neurophet (Co-CEOs Jake Junkil Been and Donghyeon Kim), an artificial intelligence (AI) solution company for brain disorders diagnosis and treatment, announced today that its software solution, Neurophet AQUA AD Plus, a comprehensive neuroimaging analysis solution for clinical evaluation related to Alzheimer's disease, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
This clearance marks Neurophet's third FDA 510(k) clearance, following Neurophet AQUA, a brain neurodegeneration imaging analysis software, and Neurophet SCALE PET, a PET image quantitative analysis software. The achievement further validates the safety and effectiveness of Neurophet's core product portfolio at a global regulatory standard.
Neurophet AQUA AD Plus is a software-based solution designed to support imaging-based clinical evaluation across the Alzheimer's disease care continuum. The software performs quantitative analysis of MRI and PET images, enabling automated labeling, visualization, volumetric quantification of brain structures and lesions, as well as standardized uptake value ratio (SUVR) analysis. Quantitative results can be compared with normative reference data to support the evaluation of neurodegeneration and cognitive impairment.
The FDA-cleared U.S. version of Neurophet AQUA AD Plus is an upgraded solution that builds upon the capabilities of Neurophet AQUA AD. Leveraging AI-based brain MRI analysis, the software automatically analyzes and quantifies:
- Hypointense lesions associated with cerebral microbleeds and superficial siderosis, and
- Hyperintense lesions related to brain edema
By providing automated lesion localization and counts, the software helps clinicians more precise assess imaging-based risk factors and supports informed, patient-specific clinical decision-making when evaluating patients undergoing Alzheimer's disease-related therapeutic management.
"Receiving FDA 510(k) clearance for Neurophet AQUA AD Plus represents a significant milestone that allows us to introduce our advanced Alzheimer's imaging technology more broadly in the U.S. market," said Jake Junkil Been, Co-CEO of Neurophet. "We plan to accelerate our expansion across the U.S. by strengthening collaborations with healthcare institutions and strategic partners."
Neurophet AQUA AD Plus was previously designated as an Innovative Medical Technology in South Korea in September 2025. Building on this momentum, Neurophet plans to progressively expand clinical adoption of the solution across global healthcare settings, further broadening its global footprint in AI-based neuroimaging.
About Neurophet
Neurophet specializes in developing solutions for diagnosis support, treatment guides, and treatment devices targeting brain disorders based on cutting-edge artificial intelligence (AI) technology. The company was founded in 2016 by Co-CEOs Jake Junkil Been and Donghyeon Kim, who developed the next-generation neuro-navigation system.
Major products include brain MRI analysis software "Neurophet AQUA", PET Image Quantitative Analysis Software "Neurophet SCALE PET", Brain imaging treatment planning software for electric and magnetic brain stimulation "Neurophet tES/TMS LAB", Alzheimer's Disease treatment prescription and monitoring software "Neurophet AQUA AD" for tracking treatment efficacy and side effects, and Multiple Sclerosis image analysis software "Neurophet AQUA MS".
Neurophet has set its top priority to helping patients suffering from brain disorders. Based on expertise in neuroscience, Neurophet will continue to challenge and grow to explore the human brain's health and pioneer solutions for brain diseases with AI technology.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Neurophet Secures FDA 510(k) Clearance for "Neurophet AQUA AD Plus," Marking Its Third U.S. Regulatory Milestone
Blending world-renowned art with early learning, the collaboration invites children to explore creativity through play
NEW YORK, Feb. 6, 2026 /PRNewswire/ -- Following their initial collaboration announcement last year, Hape Toys and The Metropolitan Museum of Art today announced that they will unveil their highly anticipated Hape x The Met toy collection exclusively at New York Toy Fair 2026, taking place February 14–17, at Booth #635.
The unique collaboration brings iconic works of art to life through beautifully crafted, developmentally rich toys designed for infants, toddlers, and young children. Drawing inspiration from The Met's world-renowned holdings, spanning 5,000 years of global art, the line transforms celebrated masterpieces into engaging play experiences that encourage curiosity, creativity, and early learning.
The collection includes wooden puzzles, stacking and building toys, sensory play items, and imaginative blocks inspired by renowned artists and artistic traditions such as Hokusai, Klimt, Monet, and Van Gogh, with works spanning The Met collection of over 2 million works of art.
"Last year, we shared our vision to merge art and early learning. This year, we are proud to see this creative concept and shared vision come to reality," said Peter Handstein, Founder and CEO of Hape. "This series reflects Hape's commitment to thoughtful design, sustainability, and child development, while introducing families to the wonder of art in a way that feels joyful, accessible, and meaningful from the very first years of life."
Each product in the collection is designed in collaboration with early-childhood experts and features museum-inspired storytelling, encouraging children to explore color, form, movement, and imagination through hands-on play. Thoughtfully designed packaging further extends the experience by sharing the story behind the artwork that inspired each toy, turning every unboxing into a cultural discovery.
"Welcoming families and inspiring the next generation of creators is central to The Met's mission," said Josh Romm, Head of Global Licensing and Partnerships at The Met. "Through our collaboration with Hape, we are extending the reach of our collection beyond the Museum's walls, transforming iconic works of art into playful, educational experiences that invite children to connect with creativity, history, and storytelling in their everyday lives."
Attendees of New York Toy Fair are invited to preview the Hape x The Met collection at Booth #635
For more information about Hape, please visit: http://toys.hape.com
For more information about The Met, please visit: https://www.metmuseum.org/
About Hape
Hape is one of the world's largest manufacturers of high-quality, eco-friendly wooden toys. Hape's mission is to promote the development of young minds through playful, innovative toys designed to inspire creativity and learning. With a focus on sustainability and craftsmanship, Hape brings educational play to families worldwide.
About The Metropolitan Museum of Art
The Metropolitan Museum of Art was founded in 1870 by a group of American citizens, businessmen and financiers as well as leading artists and thinkers of the day, who wanted to create a museum to bring art and art education to the American people. Today, The Met displays tens of thousands of objects covering 5,000 years of art from around the world for everyone to experience and enjoy. The Museum lives in two iconic sites in New York City, The Met Fifth Avenue and The Met Cloisters. Millions of people also take part in The Met experience online. Since its founding, The Met has always aspired to be more than a treasury of rare and beautiful objects. Every day, art comes alive in the Museum's galleries and through its exhibitions and events, revealing both new ideas and unexpected connections across time and across cultures.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Hape and The Metropolitan Museum of Art Unveil Art-Inspired Toy Collection at New York Toy Fair 2026

Hape and The Metropolitan Museum of Art Unveil Art-Inspired Toy Collection at New York Toy Fair 2026