DUBAI, UAE, Feb. 18, 2026 /PRNewswire/ -- The UAE SWAT Challenge 2026 has wrapped up its 7th edition in spectacular fashion, once again proving why it is regarded as the world's largest gathering of elite tactical police units.
Hosted by Dubai Police, the event brought together 109 teams from 48 countries, transforming Dubai into a global hub for specialised forces and security professionals.
Kazakhstan Dominates
After days of high intensity and demanding tactical challenges, Team Kazakhstan C claimed the overall title with 536 points. Kazakhstan A followed in second place with 515 points, while China Police C secured third with 493 points.
A Guinness World Record
For the second consecutive year, Dubai Police earned a Guinness World Record for hosting the highest number of countries participating in a specialised tactical competition, with 48 nations represented in this edition.
Rising Female Tactical Power
Eight all women teams and two mixed teams took part in this year's challenge, underscoring the expanding role of women in elite tactical operations. Their participation highlights a global shift towards greater inclusion in specialised policing roles traditionally dominated by men.
International Recognition
Robert Raines, US Consul General in Dubai and the Northern Emirates, praised the organisation and noted the steady growth of the challenge, highlighting the participation of four American teams.
Carolin Konther Lopez, Ambassador of Paraguay to the UAE, described the event as exceptionally organised and said her country's first participation reflects its commitment to strengthening international security cooperation.
A representative of Mongolia's Special Protection Agency called the challenge an outstanding platform to test operational readiness and exchange expertise with leading special units worldwide.
A Truly Global Stage
Bolivia made its debut with two specialised teams as it celebrates 200 years since the founding of its police force. The competition also featured eight women teams and two mixed teams, underscoring the growing global role of women in tactical operations.
Panama joined for the first time with a maritime airborne unit, adding further diversity to a competition that offered total prize money of 260,000 US dollars.
Advancing Readiness and Cooperation
The UAE SWAT Challenge aims to strengthen international security cooperation, enhance tactical skills and promote the exchange of best practices among specialised units worldwide. It provides a real world environment that sharpens field performance, builds mental resilience and reinforces teamwork.
Contact
UAE SWAT Challenge Media Centre
swatmedia@dubaipolice.gov.ae
+97146996224
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

48 Nations, One Arena: How Dubai United the World in Elite Tactical Showdown
| |
SANTA MARIA, Calif. and GUIPRY, France, Feb. 18, 2026 /PRNewswire/ -- In a major step forward against Antimicrobial Resistance (AMR), NG Biotech, in partnership with Hardy Diagnostics, announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designations to two rapid diagnostic assays: NG-TEST® Candida auris and NG-TEST® Acineto-5®. The designation recognizes technologies with the potential to address life-threatening conditions and significant unmet medical needs.
Both tests target pathogens classified as critical priorities by the World Health Organization (WHO). Candida auris, listed in the WHO Fungal Priority Pathogens List (2022), is a multidrug-resistant yeast responsible for hospital outbreaks worldwide. It is often difficult to detect and associated with high mortality. Carbapenem-resistant Acinetobacter baumannii (CRAB), included in the WHO Bacterial Priority Pathogens List (2024), is among the most dangerous hospital-acquired bacteria due to its resistance profile and rapid transmission in healthcare settings.
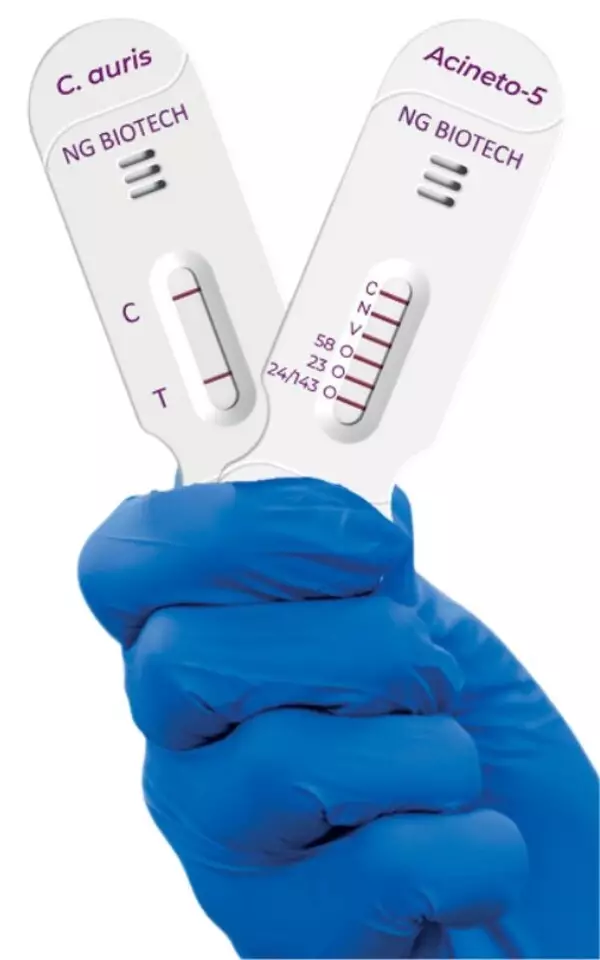
NG-TEST® Candida auris is the first rapid lateral flow immunoassay specifically designed to identify C. auris from cultured samples in 15 minutes. Published data demonstrate 100% concordance with reference methods across diverse isolates, supporting its role in outbreak investigation and infection control.
NG-TEST® Acineto-5® detects and differentiates five major carbapenemase families—OXA-23-like, OXA-24/143-like, OXA-58-like, VIM, and NDM—directly from Acinetobacter samples, also delivering results within 15 minutes. The PCR-free assay is designed for ease of use, without specialized equipment.
"These breakthrough designations validate both the technology behind our assays and the real-world need they address," said Milovan Stankov-Pugès, CEO, NG Biotech.
"The designation underscores the growing urgency around rapid detection of multidrug-resistant organisms that pose serious risks in healthcare settings," said Andre Hsiung, Chief Scientific Officer of Hardy Diagnostics.
Developed and manufactured in France by NG Biotech, the assays are distributed exclusively in the United States by Hardy Diagnostics. They are currently available for Research Use Only while FDA review continues.
By accelerating detection of high-risk pathogens, these breakthrough-designated tests aim to strengthen surveillance, guide infection control decisions, and support global efforts to fight antimicrobial resistance.
Learn more about NG Biotech
Learn more about Hardy Diagnostics
Media & Contact Information
NG BIOTECH
Atelier Relais Le Tremplin
Parc d'Activités de Courbouton, Secteur 1
35480 Guipry, France
+33 (0)2 23 30 17 83
Ms. Pauline Cognet
p.cognet@ngbiotech.com
www.ngbiotech.com
HARDY DIAGNOSTICS
1430 West McCoy Lane
Santa Maria, CA 93455, USA
(805) 346-2766 ext. 5598
Ms. Megan Maloney Roesner
RoesnerM@hardydiagnostics.com
www.HardyDiagnostics.com
SANTA MARIA, Calif. and GUIPRY, France, Feb. 18, 2026 /PRNewswire/ -- In a major step forward against Antimicrobial Resistance (AMR), NG Biotech, in partnership with Hardy Diagnostics, announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designations to two rapid diagnostic assays: NG-TEST® Candida auris and NG-TEST® Acineto-5®. The designation recognizes technologies with the potential to address life-threatening conditions and significant unmet medical needs.
Both tests target pathogens classified as critical priorities by the World Health Organization (WHO). Candida auris, listed in the WHO Fungal Priority Pathogens List (2022), is a multidrug-resistant yeast responsible for hospital outbreaks worldwide. It is often difficult to detect and associated with high mortality. Carbapenem-resistant Acinetobacter baumannii (CRAB), included in the WHO Bacterial Priority Pathogens List (2024), is among the most dangerous hospital-acquired bacteria due to its resistance profile and rapid transmission in healthcare settings.
NG-TEST® Candida auris is the first rapid lateral flow immunoassay specifically designed to identify C. auris from cultured samples in 15 minutes. Published data demonstrate 100% concordance with reference methods across diverse isolates, supporting its role in outbreak investigation and infection control.
NG-TEST® Acineto-5® detects and differentiates five major carbapenemase families—OXA-23-like, OXA-24/143-like, OXA-58-like, VIM, and NDM—directly from Acinetobacter samples, also delivering results within 15 minutes. The PCR-free assay is designed for ease of use, without specialized equipment.
"These breakthrough designations validate both the technology behind our assays and the real-world need they address," said Milovan Stankov-Pugès, CEO, NG Biotech.
"The designation underscores the growing urgency around rapid detection of multidrug-resistant organisms that pose serious risks in healthcare settings," said Andre Hsiung, Chief Scientific Officer of Hardy Diagnostics.
Developed and manufactured in France by NG Biotech, the assays are distributed exclusively in the United States by Hardy Diagnostics. They are currently available for Research Use Only while FDA review continues.
By accelerating detection of high-risk pathogens, these breakthrough-designated tests aim to strengthen surveillance, guide infection control decisions, and support global efforts to fight antimicrobial resistance.
Learn more about NG Biotech
Learn more about Hardy Diagnostics
Media & Contact Information
NG BIOTECH
Atelier Relais Le Tremplin
Parc d'Activités de Courbouton, Secteur 1
35480 Guipry, France
+33 (0)2 23 30 17 83
Ms. Pauline Cognet
p.cognet@ngbiotech.com
www.ngbiotech.com
HARDY DIAGNOSTICS
1430 West McCoy Lane
Santa Maria, CA 93455, USA
(805) 346-2766 ext. 5598
Ms. Megan Maloney Roesner
RoesnerM@hardydiagnostics.com
www.HardyDiagnostics.com
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
FDA Grants Breakthrough Device Designation to Two Rapid Tests Targeting Critical Drug-Resistant Pathogens











