|
- More than 80% relative bioavailability compared with injectable obesity therapy, well above the roughly 30% reported for existing microneedle patches and setting a new global benchmark
- A potential game changer that addresses both the pain of injections and the low efficiency of oral formulations
- In the obesity treatment market, delivery technology is expected to be the decisive factor shaping future leaders
SEOUL, South Korea, Aug. 13, 2025 /PRNewswire/ -- Daewoong Pharmaceutical (Co-CEOs Seong-Soo Park and Chang-Jae Lee) and Daewoong Therapeutics (CEO Bok-Ki Kang) announced that their proprietary semaglutide microneedle patch achieved more than 80%relative bioavailability compared to the injectable formulation in a pilot human pharmacokinetic study.
This was based on the healthy volunteer study using semaglutide microneedle patch using Daewoong Therapeutics' CLOPAM® (CLOsed Packed Aero-pressured Microneedle) drug-delivery platform. The study directly compared delivery efficiency with semaglutide subcutaneous injection.
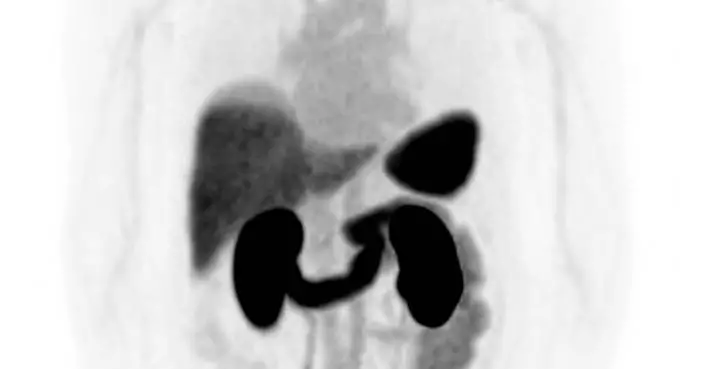
In the trial, 70 healthy adults received single dose of either a semaglutide microneedle patch or a subcutaneous injection. Plasma concentrations were measured and then compared with results from semaglutide subcutaneous injection under the same conditions. After adjusting to equivalent dose, the patch showed more than 80%relative bioavailability compared with injection.
This result is the highest reported for a microneedle patch with the same active ingredient, far exceeding the approximately 30 percent achieved by existing patches. It also showed around 160 times higher bioavailability than semaglutide oral tablet.
Therapeutic plasma levels were maintained for one week, supporting the potential for once-weekly dosing with a single high-load patch. The exposure profile was comparable to that of the injectable counterpart, which strengthens the commercial prospects for a patch-based obesity treatment.
Technology as the Decisive Factor in the Obesity Market
According to Grand View Research, the global obesity therapeutics market is valued at 15.9 billion US dollars in 2024 and is projected to grow to 60.5 billion US dollars by 2030. Currently, therapeutic and safety advantages in the GLP-1 drug class are limited, and experts expect Experts expect delivery technologies improving both bioavailability and patient convenience will be the factor that determines future market leaders. Microneedle patches are attracting attention because they address two key limitations of current therapies: pain of injections and low drug delivery efficiency of oral formulations.
While semaglutide oral tablet is available on the market, it has very low bioavailability and requires complex medication regimen, such as fasting before dosing, limiting water intake, and avoiding co-administration with other drugs for chronic patients potentially lowering adherence. A once-weekly microneedle patch offers a simpler, needle-free option that could be used by a wider range of patients, including those who avoid injections.
Daewoong's Microneedle Technology Innovation Leading the Global Market
The results were made possible by Daewoong Therapeutics' patented CLOPAM® microneedle platform technology, a dissolving-type microneedle technology designed for precise, skin-based drug delivery. The platform uses pressurized drying and hermetic packaging to improve drug uniformity and stability, allowing accurate dosing with minimal contamination risk. CLOPAM® currently has 52 patent filings worldwide and is positioning to be one of Daewoong's leading technologies.
Unlike other injectable products containing semaglutide that require cold-chain storage, Daewoong's patches remain stable at room temperature, which can reduce distribution costs. They also help cut down on medical waste from syringes and needles, aligning with sustainability goals in healthcare.
Based on these results, Daewoong Therapeutics is planning to broaden the collaborative initiatives to the global market via technology licensing, co-development, and out-licensing to prepare for commercialization.
"The microneedle patch must achieve not only high bioavailability but also a drug load sufficient for therapeutic effect," said Bok-Ki Kang, CEO of Daewoong Therapeutics. "In this study, we incorporated a high dose of semaglutide into a single patch and showed the potential for once-weekly application. This overcomes a key limitation of previous microneedle approaches and provides a strong foundation for global technology transfer and commercialization."
"Microneedle formats have long promised better adherence, but high-dose delivery has been a challenge," said Seong-Soo Park, CEO of Daewoong Pharmaceutical. "This work is the first to overcome that barrier. We plan to expand microneedle technology to a wide range of biologics to lead the global platform market."
About Daewoong Pharmaceutical
Daewoong Pharmaceutical (KRX: 069620.KS), established in 1945, is a global pharmaceutical company headquartered in South Korea. The company develops, manufactures, and markets innovative medicines and healthcare solutions with the mission to improve quality of life worldwide. Daewoong specializes in treatments for intractable and rare diseases, supported by in-house research and development, open innovation, and advanced manufacturing capabilities.
Recent achievements include the development and approval of novel drugs for gastroesophageal reflux disease (Fexuprazan) and Type 2 diabetes (Enavogliflozin) in consecutive years. The company is also developing a first-in-class oral anti-fibrotic agent for idiopathic pulmonary fibrosis (Bersiporocin), which has received both orphan drug designation and Fast Track status from the US FDA.
About Daewoong Therapeutics
Daewoong Therapeutics Inc., is a subsidiary of Daewoong Group located in South Korea. The company is focused on developing novel drug delivery systems including the dissolving microneedle platform, CLOPAM®. CLOPAM® is currently being applied to various fields including obesity/metabolic diseases and others with aims to improve patient convenience and medication adherence.
- More than 80% relative bioavailability compared with injectable obesity therapy, well above the roughly 30% reported for existing microneedle patches and setting a new global benchmark
- A potential game changer that addresses both the pain of injections and the low efficiency of oral formulations
- In the obesity treatment market, delivery technology is expected to be the decisive factor shaping future leaders
SEOUL, South Korea, Aug. 13, 2025 /PRNewswire/ -- Daewoong Pharmaceutical (Co-CEOs Seong-Soo Park and Chang-Jae Lee) and Daewoong Therapeutics (CEO Bok-Ki Kang) announced that their proprietary semaglutide microneedle patch achieved more than 80%relative bioavailability compared to the injectable formulation in a pilot human pharmacokinetic study.
This was based on the healthy volunteer study using semaglutide microneedle patch using Daewoong Therapeutics' CLOPAM® (CLOsed Packed Aero-pressured Microneedle) drug-delivery platform. The study directly compared delivery efficiency with semaglutide subcutaneous injection.
In the trial, 70 healthy adults received single dose of either a semaglutide microneedle patch or a subcutaneous injection. Plasma concentrations were measured and then compared with results from semaglutide subcutaneous injection under the same conditions. After adjusting to equivalent dose, the patch showed more than 80%relative bioavailability compared with injection.
This result is the highest reported for a microneedle patch with the same active ingredient, far exceeding the approximately 30 percent achieved by existing patches. It also showed around 160 times higher bioavailability than semaglutide oral tablet.
Therapeutic plasma levels were maintained for one week, supporting the potential for once-weekly dosing with a single high-load patch. The exposure profile was comparable to that of the injectable counterpart, which strengthens the commercial prospects for a patch-based obesity treatment.
Technology as the Decisive Factor in the Obesity Market
According to Grand View Research, the global obesity therapeutics market is valued at 15.9 billion US dollars in 2024 and is projected to grow to 60.5 billion US dollars by 2030. Currently, therapeutic and safety advantages in the GLP-1 drug class are limited, and experts expect Experts expect delivery technologies improving both bioavailability and patient convenience will be the factor that determines future market leaders. Microneedle patches are attracting attention because they address two key limitations of current therapies: pain of injections and low drug delivery efficiency of oral formulations.
While semaglutide oral tablet is available on the market, it has very low bioavailability and requires complex medication regimen, such as fasting before dosing, limiting water intake, and avoiding co-administration with other drugs for chronic patients potentially lowering adherence. A once-weekly microneedle patch offers a simpler, needle-free option that could be used by a wider range of patients, including those who avoid injections.
Daewoong's Microneedle Technology Innovation Leading the Global Market
The results were made possible by Daewoong Therapeutics' patented CLOPAM® microneedle platform technology, a dissolving-type microneedle technology designed for precise, skin-based drug delivery. The platform uses pressurized drying and hermetic packaging to improve drug uniformity and stability, allowing accurate dosing with minimal contamination risk. CLOPAM® currently has 52 patent filings worldwide and is positioning to be one of Daewoong's leading technologies.
Unlike other injectable products containing semaglutide that require cold-chain storage, Daewoong's patches remain stable at room temperature, which can reduce distribution costs. They also help cut down on medical waste from syringes and needles, aligning with sustainability goals in healthcare.
Based on these results, Daewoong Therapeutics is planning to broaden the collaborative initiatives to the global market via technology licensing, co-development, and out-licensing to prepare for commercialization.
"The microneedle patch must achieve not only high bioavailability but also a drug load sufficient for therapeutic effect," said Bok-Ki Kang, CEO of Daewoong Therapeutics. "In this study, we incorporated a high dose of semaglutide into a single patch and showed the potential for once-weekly application. This overcomes a key limitation of previous microneedle approaches and provides a strong foundation for global technology transfer and commercialization."
"Microneedle formats have long promised better adherence, but high-dose delivery has been a challenge," said Seong-Soo Park, CEO of Daewoong Pharmaceutical. "This work is the first to overcome that barrier. We plan to expand microneedle technology to a wide range of biologics to lead the global platform market."
About Daewoong Pharmaceutical
Daewoong Pharmaceutical (KRX: 069620.KS), established in 1945, is a global pharmaceutical company headquartered in South Korea. The company develops, manufactures, and markets innovative medicines and healthcare solutions with the mission to improve quality of life worldwide. Daewoong specializes in treatments for intractable and rare diseases, supported by in-house research and development, open innovation, and advanced manufacturing capabilities.
Recent achievements include the development and approval of novel drugs for gastroesophageal reflux disease (Fexuprazan) and Type 2 diabetes (Enavogliflozin) in consecutive years. The company is also developing a first-in-class oral anti-fibrotic agent for idiopathic pulmonary fibrosis (Bersiporocin), which has received both orphan drug designation and Fast Track status from the US FDA.
About Daewoong Therapeutics
Daewoong Therapeutics Inc., is a subsidiary of Daewoong Group located in South Korea. The company is focused on developing novel drug delivery systems including the dissolving microneedle platform, CLOPAM®. CLOPAM® is currently being applied to various fields including obesity/metabolic diseases and others with aims to improve patient convenience and medication adherence.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Daewoong Therapeutics Microneedle Patch Achieves Best-in-Class Bioavailability, Proving the Strength of Its Drug-Delivery Platform
ALULA, Saudi Arabia, Jan. 16, 2026 /PRNewswire/ -- Today marks the official opening of Desert X AlUla 2026, the landmark fourth edition of the international, open-air biennial exhibition. Arts AlUla, in collaboration with Desert X, welcomes visitors to experience a stellar line-up of Saudi and international multi-generational artists whose site-responsive earthworks, sculptures, and installations will engage in a powerful dialogue with the awe-inspiring landscapes and layered heritage of AlUla.
As a premier destination rich in ancient history and breathtaking nature, AlUla, located in Northwest Saudi Arabia, solidifies its position on the global stage as a dynamic, emerging land art destination with Desert X AlUla, the region's first public art biennale, and a key highlight of the AlUla Arts Festival.
The 2026 edition of Desert X AlUla brings together 11 acclaimed artists whose diverse works reflect a wide spectrum of ideas, materials, and traditions. From monumental kinetic sculpture to sound-based explorations above and below ground, each commission is deeply rooted in relationships to AlUla's distinctive environment, further cementing Desert X AlUla's reputation as a globally significant platform for site-responsive land art.
Desert X AlUla runs until February 28, 2026, as a cornerstone of the annual AlUla Arts Festival. Curated by Wejdan Reda, Zoé Whitley, with artistic direction by Neville Wakefield, and Raneem Farsi, its fourth edition explores 'Space Without Measure.' Inspired by Kahlil Gibran, the theme fosters contemplation of imagination within AlUla's natural settings. The exhibition, set in the desert canyons of AlUla, serves as a pre-opening programme for Wadi AlFann, offering a pivotal glimpse into AlUla's plans to create a permanent land art 'Valley of the Arts.'
Hamad Alhomiedan, Director of Arts & Creative Industries at the Royal Commission for AlUla (RCU), said: "At Desert X AlUla 2026, audiences will engage with art that deeply converses with AlUla's unique landscapes and rich heritage. These compelling commissions highlight AlUla's dynamic transformation into a major global destination, where ancient and contemporary expressions converge. This exhibition is part of our broader revitalisation of AlUla as a culturally rich destination to live, work and visit and integral to positioning AlUla in the global dialogue of contemporary art and as a precursor to monumental projects like Wadi AlFann."
Participating artist/artworks are:
- Sara Abdu, A Kingdom Where No One Dies: Contours of Resonance
- Mohammad Alfaraj, What was the Question Again?
- Mohammed AlSaleem, The Thorn, AlShuruf Unit, The Triangles, Flower Bud, and Al Ahilla (courtesy of Royal Commission for Riyadh City)
- Tarek Atoui, The Water Song
- Bahraini-Danish, Bloom
- Maria Magdalena Campos-Pons, Imole Red
- Agnes Denes, The Living Pyramid
- Ibrahim El-Salahi, Haraza Tree
- Basmah Felemban, Murmur of Pebbles
- Vibha Galhotra, Future Fables
- Héctor Zamora, Tar HyPar
For further information, please contact:
Sabrine.Shaw@bursonglobal.com
AlUlaArtsFestival@bursonglobal.com
Multimedia gallery:
High-resolution photos of all 11 artists and their artworks can be found here.
About AlUla and Arts AlUla
Located 1,100 km from Riyadh, in North-West Saudi Arabia, AlUla is a place of extraordinary natural and human heritage. The vast area, covering 22,561km², includes a lush oasis valley, towering sandstone mountains and ancient cultural heritage sites dating back thousands of years to when the Lihyan and Nabataean kingdoms reigned.
The most well-known and recognised site in AlUla is Hegra, the principal southern city of the Nabataean Kingdom and Saudi Arabia's first UNESCO World Heritage Site. AlUla is also home to ancient Dadan, the capital of the Dadan and Lihyan Kingdoms and considered to be one of the most developed 1st millennium BCE cities of the Arabian Peninsula, and Jabal Ikmah, an open air library of hundreds of inscriptions and writings in many different languages. AlUla Old Town Village, a labyrinth of more than 900 mudbrick homes was developed from at least the 12th century and has been revitalised as the vibrant hub for visitors and residents.
The creation of Arts AlUla within The Royal Commission for AlUla (RCU) is a commitment to crafting the next chapters in a millennia of artistic creation – celebrating cultural inheritance and shaping a future inspired by artists built be artists. The work of Arts AlUla seeks to preserve this legacy: fuse the old with the new; the local with the international, keeping the arts central to the spirit of AlUla as a place of extraordinary natural and human heritage.
Wadi AlFann, meaning 'Valley of the Arts,' will be a global cultural destination for land art, unveiling from 2028 onwards, where era-defining works by artists from around the world will be permanently sited in the monumental landscape of AlUla, the extraordinary desert region of north-west Saudi Arabia.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Desert X AlUla 2026: monumental land art exhibition opens in the ancient oasis of AlUla