|
- FDA registration fulfills requirements for U.S. market entry
- Initial shipments to Europe and Latin America to begin with pre-approved countries
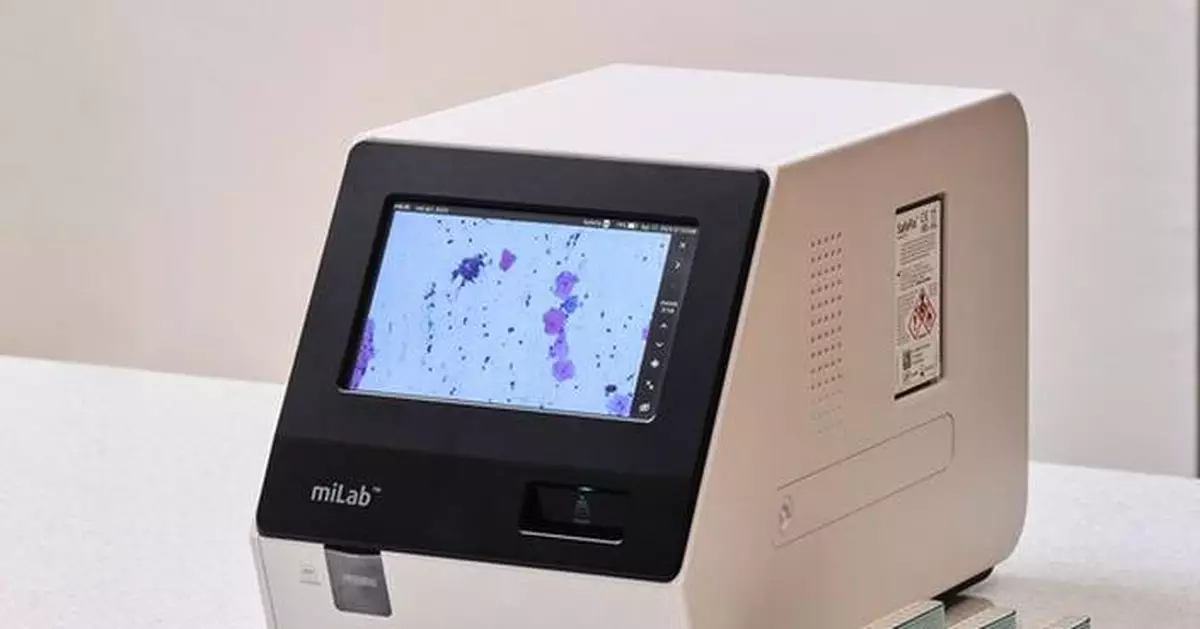
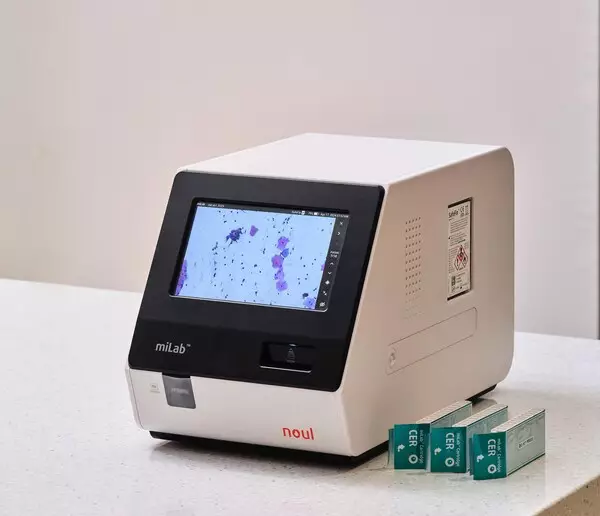
YONGIN, South Korea, Sept. 23, 2025 /PRNewswire/ -- Noul Co., Ltd. (CEO: David Lim), a medical AI company specializing in blood and cancer diagnostics, announced that FDA registration has been completed for its cervical cancer diagnostic cartridge miLab™ Cartridge CER and clearing agent SafeFix™ CER, both core components of the company's cervical cancer diagnostic solution miLab™ CER.
With this registration, Noul has fulfilled the minimum regulatory requirements for entry into the U.S. market. Beginning in October, the company will launch commercial shipments of miLab CER in Europe and Latin America, starting with countries where regulatory clearance has already been obtained.
In addition to its existing miLab platform for malaria diagnostics and blood analysis, this FDA device listing provides a foothold for Noul to enter the U.S. market with its cervical cancer portfolio. The company is currently preparing phased FDA 510(k) submissions for disease-specific analysis software to be integrated into the miLab platform.
CEO David Lim stated "The registration of our miLab CER product line marks an important milestone for entering the U.S. market and a catalyst for our global expansion." He added, "In parallel with the upcoming launches in Europe and Latin America, shipments will begin this month to countries including Qatar, Panama, and the UK, where regulatory approvals are already in place."
Cervical cancer survival rates highlight the critical need for early detection: in the U.S., the five-year survival rate exceeds 90% when diagnosed early but drops below 20% at late stages.Screening disparities remain stark, with uninsured and low-income women participating at rates more than 20% lower than average, and African American women facing a 60% higher incidence and more than double the mortality rate compared to white women.
Earlier this year, Noul secured global supply agreements in six Latin American countries, including Panama, as well as Qatar, prior to launch. The company has since obtained registration in Switzerland and regulatory clearance in Vietnam, building momentum for broader international expansion. With proven demand in Europe and Latin America, Noul now aims to accelerate adoption in the U.S. market.
Noul's miLab CER automates cervical cytology with an integrated AI-powered system that performs sample preparation, imaging, and analysis. The product was named one of the three recommended technologies for cervical cancer triage — alongside Roche and Hologic — in the 2024 WHO–Unitaid report.
About Noul Co., Ltd.
"Enabling blood and cancer diagnostics anywhere in the world with AI."
Founded in December 2015, Noul (376930.KQ) is an on-device AI healthcare company with a mission to explore global challenges that threaten human health and life, discover novel solutions, and realize those possibilities. Noul has commercialized the world's first AI-powered diagnostic lab, miLab™, through convergence of AI, biotechnology, and compact robotics.
The miLab™ Platform is the only solution that fully automates the microscopic diagnostic process—from sample preparation to AI-based image analysis—delivering lab-grade blood and cancer diagnostics at the point of care. Within 15 minutes, miLab™ produces precise results even in low-resource settings.
In 2022, miLab™ was described in a UNITAID report as "The most advanced digital microscope and fully integrated bench-top platform" for malaria diagnostics. It is now deployed in 28 countries, used by pharmaceutical companies, hospitals, labs, and public health institutions worldwide.
With strong clinical validation and proven performance, Noul is rapidly expanding its market presence, especially in malaria, blood analysis, and cervical cancer diagnostics. The company's vision is to make a meaningful impact on the lives of 1 billion people worldwide through accessible, innovative diagnostic technologies.
Website: https://noul.com
- FDA registration fulfills requirements for U.S. market entry
- Initial shipments to Europe and Latin America to begin with pre-approved countries
YONGIN, South Korea, Sept. 23, 2025 /PRNewswire/ -- Noul Co., Ltd. (CEO: David Lim), a medical AI company specializing in blood and cancer diagnostics, announced that FDA registration has been completed for its cervical cancer diagnostic cartridge miLab™ Cartridge CER and clearing agent SafeFix™ CER, both core components of the company's cervical cancer diagnostic solution miLab™ CER.
With this registration, Noul has fulfilled the minimum regulatory requirements for entry into the U.S. market. Beginning in October, the company will launch commercial shipments of miLab CER in Europe and Latin America, starting with countries where regulatory clearance has already been obtained.
In addition to its existing miLab platform for malaria diagnostics and blood analysis, this FDA device listing provides a foothold for Noul to enter the U.S. market with its cervical cancer portfolio. The company is currently preparing phased FDA 510(k) submissions for disease-specific analysis software to be integrated into the miLab platform.
CEO David Lim stated "The registration of our miLab CER product line marks an important milestone for entering the U.S. market and a catalyst for our global expansion." He added, "In parallel with the upcoming launches in Europe and Latin America, shipments will begin this month to countries including Qatar, Panama, and the UK, where regulatory approvals are already in place."
Cervical cancer survival rates highlight the critical need for early detection: in the U.S., the five-year survival rate exceeds 90% when diagnosed early but drops below 20% at late stages.Screening disparities remain stark, with uninsured and low-income women participating at rates more than 20% lower than average, and African American women facing a 60% higher incidence and more than double the mortality rate compared to white women.
Earlier this year, Noul secured global supply agreements in six Latin American countries, including Panama, as well as Qatar, prior to launch. The company has since obtained registration in Switzerland and regulatory clearance in Vietnam, building momentum for broader international expansion. With proven demand in Europe and Latin America, Noul now aims to accelerate adoption in the U.S. market.
Noul's miLab CER automates cervical cytology with an integrated AI-powered system that performs sample preparation, imaging, and analysis. The product was named one of the three recommended technologies for cervical cancer triage — alongside Roche and Hologic — in the 2024 WHO–Unitaid report.
About Noul Co., Ltd.
"Enabling blood and cancer diagnostics anywhere in the world with AI."
Founded in December 2015, Noul (376930.KQ) is an on-device AI healthcare company with a mission to explore global challenges that threaten human health and life, discover novel solutions, and realize those possibilities. Noul has commercialized the world's first AI-powered diagnostic lab, miLab™, through convergence of AI, biotechnology, and compact robotics.
The miLab™ Platform is the only solution that fully automates the microscopic diagnostic process—from sample preparation to AI-based image analysis—delivering lab-grade blood and cancer diagnostics at the point of care. Within 15 minutes, miLab™ produces precise results even in low-resource settings.
In 2022, miLab™ was described in a UNITAID report as "The most advanced digital microscope and fully integrated bench-top platform" for malaria diagnostics. It is now deployed in 28 countries, used by pharmaceutical companies, hospitals, labs, and public health institutions worldwide.
With strong clinical validation and proven performance, Noul is rapidly expanding its market presence, especially in malaria, blood analysis, and cervical cancer diagnostics. The company's vision is to make a meaningful impact on the lives of 1 billion people worldwide through accessible, innovative diagnostic technologies.
Website: https://noul.com
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Noul Secures FDA Registration for Cervical Cancer Diagnostic Cartridge
ALULA, Saudi Arabia, Jan. 16, 2026 /PRNewswire/ -- Today marks the official opening of Desert X AlUla 2026, the landmark fourth edition of the international, open-air biennial exhibition. Arts AlUla, in collaboration with Desert X, welcomes visitors to experience a stellar line-up of Saudi and international multi-generational artists whose site-responsive earthworks, sculptures, and installations will engage in a powerful dialogue with the awe-inspiring landscapes and layered heritage of AlUla.
As a premier destination rich in ancient history and breathtaking nature, AlUla, located in Northwest Saudi Arabia, solidifies its position on the global stage as a dynamic, emerging land art destination with Desert X AlUla, the region's first public art biennale, and a key highlight of the AlUla Arts Festival.
The 2026 edition of Desert X AlUla brings together 11 acclaimed artists whose diverse works reflect a wide spectrum of ideas, materials, and traditions. From monumental kinetic sculpture to sound-based explorations above and below ground, each commission is deeply rooted in relationships to AlUla's distinctive environment, further cementing Desert X AlUla's reputation as a globally significant platform for site-responsive land art.
Desert X AlUla runs until February 28, 2026, as a cornerstone of the annual AlUla Arts Festival. Curated by Wejdan Reda, Zoé Whitley, with artistic direction by Neville Wakefield, and Raneem Farsi, its fourth edition explores 'Space Without Measure.' Inspired by Kahlil Gibran, the theme fosters contemplation of imagination within AlUla's natural settings. The exhibition, set in the desert canyons of AlUla, serves as a pre-opening programme for Wadi AlFann, offering a pivotal glimpse into AlUla's plans to create a permanent land art 'Valley of the Arts.'
Hamad Alhomiedan, Director of Arts & Creative Industries at the Royal Commission for AlUla (RCU), said: "At Desert X AlUla 2026, audiences will engage with art that deeply converses with AlUla's unique landscapes and rich heritage. These compelling commissions highlight AlUla's dynamic transformation into a major global destination, where ancient and contemporary expressions converge. This exhibition is part of our broader revitalisation of AlUla as a culturally rich destination to live, work and visit and integral to positioning AlUla in the global dialogue of contemporary art and as a precursor to monumental projects like Wadi AlFann."
Participating artist/artworks are:
- Sara Abdu, A Kingdom Where No One Dies: Contours of Resonance
- Mohammad Alfaraj, What was the Question Again?
- Mohammed AlSaleem, The Thorn, AlShuruf Unit, The Triangles, Flower Bud, and Al Ahilla (courtesy of Royal Commission for Riyadh City)
- Tarek Atoui, The Water Song
- Bahraini-Danish, Bloom
- Maria Magdalena Campos-Pons, Imole Red
- Agnes Denes, The Living Pyramid
- Ibrahim El-Salahi, Haraza Tree
- Basmah Felemban, Murmur of Pebbles
- Vibha Galhotra, Future Fables
- Héctor Zamora, Tar HyPar
For further information, please contact:
Sabrine.Shaw@bursonglobal.com
AlUlaArtsFestival@bursonglobal.com
Multimedia gallery:
High-resolution photos of all 11 artists and their artworks can be found here.
About AlUla and Arts AlUla
Located 1,100 km from Riyadh, in North-West Saudi Arabia, AlUla is a place of extraordinary natural and human heritage. The vast area, covering 22,561km², includes a lush oasis valley, towering sandstone mountains and ancient cultural heritage sites dating back thousands of years to when the Lihyan and Nabataean kingdoms reigned.
The most well-known and recognised site in AlUla is Hegra, the principal southern city of the Nabataean Kingdom and Saudi Arabia's first UNESCO World Heritage Site. AlUla is also home to ancient Dadan, the capital of the Dadan and Lihyan Kingdoms and considered to be one of the most developed 1st millennium BCE cities of the Arabian Peninsula, and Jabal Ikmah, an open air library of hundreds of inscriptions and writings in many different languages. AlUla Old Town Village, a labyrinth of more than 900 mudbrick homes was developed from at least the 12th century and has been revitalised as the vibrant hub for visitors and residents.
The creation of Arts AlUla within The Royal Commission for AlUla (RCU) is a commitment to crafting the next chapters in a millennia of artistic creation – celebrating cultural inheritance and shaping a future inspired by artists built be artists. The work of Arts AlUla seeks to preserve this legacy: fuse the old with the new; the local with the international, keeping the arts central to the spirit of AlUla as a place of extraordinary natural and human heritage.
Wadi AlFann, meaning 'Valley of the Arts,' will be a global cultural destination for land art, unveiling from 2028 onwards, where era-defining works by artists from around the world will be permanently sited in the monumental landscape of AlUla, the extraordinary desert region of north-west Saudi Arabia.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

Desert X AlUla 2026: monumental land art exhibition opens in the ancient oasis of AlUla