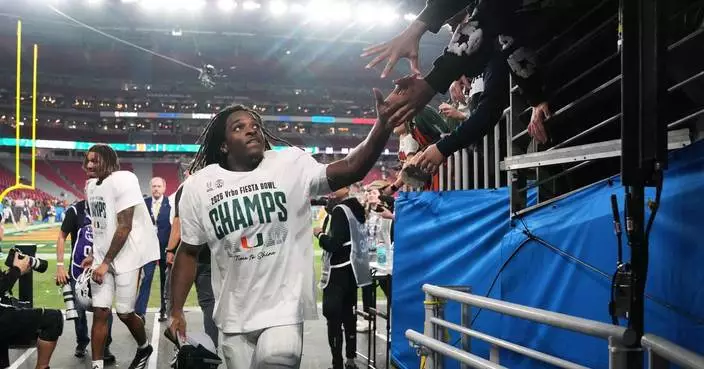FARMINGDALE, N.Y. (AP) — Rory McIlroy still remembers his tears from losing so badly in the Ryder Cup. What stung even more were the words from that Sunday four years ago at Whistling Straits.
The Americans won 19-9, the biggest Ryder Cup blowout ever over Europe. Yes, the gallery was one-sided because of travel restrictions from the COVID-19 pandemic. And yes, that was a powerful U.S. team with all 12 playing at a high level. That never seems to happen.
Click to Gallery
United States captain Keegan Bradley reacts on the second hole at Bethpage Black golf course during the Ryder Cup golf tournament, Saturday, Sept. 27, 2025, in Farmingdale, N.Y. (AP Photo/Robert Bukaty)
Europe captain Luke Donald poses with the trophy after winning the Ryder Cup golf tournament against the United States on the Bethpage Black golf course, Sunday, Sept. 28, 2025, in Farmingdale, N.Y. (AP Photo/Lindsey Wasson)
Europe's Shane Lowry celebrates after making the winning putt putt on the 18th hole during their singles match on the Bethpage Black golf course at the Ryder Cup golf tournament, Sunday, Sept. 28, 2025, in Farmingdale, N.Y. (AP Photo/Matt Slocum)
Europe's Rory McIlroy poses with the trophy after winning the Ryder Cup golf tournament against the United States on the Bethpage Black golf course, Sunday, Sept. 28, 2025, in Farmingdale, N.Y. (AP Photo/Seth Wenig)
Europe's Shane Lowry and Justin Rose celebrate with the trophy after winning the Ryder Cup golf tournament against the United States on the Bethpage Black golf course, Sunday, Sept. 28, 2025, in Farmingdale, N.Y. (AP Photo/Robert Bukaty)
This was a team that was going to change the course of the Ryder Cup.
“I was trying to tell the guys, ‘Let’s get to 20 points,' because this is going to be the next era of Ryder Cup teams for the U.S. side,” Patrick Cantlay said that day.
“If we play like we did this week, the score will be the same over there,” Jordan Spieth added.
McIlroy couldn't help but think of those predictions as Europe celebrated its second straight win since that beating, hanging on for a 15-13 victory.
He looked around at flags draped around each teammate from their nine countries, all of them mostly proud of the Team Europe emblem on the crest of their shirts. Unseen was the image of Seve Ballesteros stitched in the inside of the shirt so that it touched their hearts.
“The comments and what people were saying after Whistling Straits about the decades of American dominance, we took a lot from that,” McIlroy said. “We let that fuel us.”
The “American dominance” ended six years after continental Europe was invited to the party, and there is little to suggest that's about to change.
Playing on the road against an angry New York crowd that was nasty and disruptive only fueled Europe even more. It set a Ryder Cup record — under the current format that dates to 1979 — by losing only four of the 16 team matches going into Sunday.
No team had ever come back from more than a four-point deficit. Europe was up by seven.
It was close in the end — too close for Europe, until Shane Lowry came through with biggest putt of his life to secure the gold trophy — but this requires a bigger picture.
McIlroy has been saying for the last two years winning a Ryder Cup on the road is among the biggest accomplishments in golf. He must have been referring to the Americans, who haven't done that since 1993.
Europe picked up its fifth road win in the last 10 Ryder Cups, and it now has won 11 of the last 15 times. That's the very definition of dominance.
“When you think about the last away Ryder Cup about what people were saying about decades of American dominance — whether it was home for them or away — and to be able to do what we’ve done in Rome and then here, you know, it shut a lot of people up,” McIlroy said.
Whistling Straits, and even the U.S. win at Hazeltine before that in 2016, is starting to look like a blip on the radar instead of a foundation on which to build.
Captain Luke Donald was told Europe had dominated the last decade and was in position to do that for the following decade. That's when Lowry interject, “You guys told us we wouldn't win one for 20 years, though.”
The Americans indeed put a scare into Europe, but even that required all the magic it could muster. Cameron Young and Justin Thomas both had to make 12-foot birdie putt on the 18th hole to win their matches.
Eight of the singles matches went to the 18th hole, the most in the Ryder Cup since eight matches in 1993. In both years, one European didn't play and an American whose name was in the envelope — Lanny Wadkins in 1993, Harris English this time — were given a halve.
Perhaps what the Americans didn't see coming after their big win at Whistling Straits was a changing of the guard. Lowry, Tyrrell Hatton and Viktor Hovland were Ryder Cup rookies. Jon Rahm, Matt Fitzpatrick and and Tommy Fleetwood were playing in their second.
They were a combined 13-5-5 at Bethpage Black.
And then there's the Donald factor — the European captain, not the Ryder Cup guest on Friday who had Air Force One fly over the 15th fairway.
He had been left behind when Europe took Henrik Stenson for the 2023 matches in Rome. And then Stenson went to LIV Golf and was stripped of his captaincy, Donald had only 13 months to prepare and he's pushed all the right buttons ever since.
U.S. captain Keegan Bradley referred to Donald as the “best European captain of all time.”
“He won home and away, and he won a Ryder Cup in New York at Bethpage,” Bradley said. “He turned this European team into a really unstoppable force, especially the first two day. ... He put his team in the best position to win.”
How close was it? Even after the great American rally, Europe only needed a halve from the six matches still on the course to capture the cup. There were no European blue scores on the board, but all the matches were tight.
Lowry got the job done, with help from Russell Henley, who twice left 10-foot birdie putts short on the 17th and 18th holes that would have won him the match. Hatton let Collin Morikawa off the hook by missing three straight birdie chances from 8, 5 and 12 feet. But he never in big trouble and wound up getting another halve that made Europe an outright winner.
And so it's on to Ireland for 2027, a chance for the Americans to end 34 years of winning away from home, and end six years without winning the Ryder Cup. The 17-inch chalice goes back to the UK, a trophy the Americans still seem to only borrow once in a while.
AP Ryder Cup coverage: https://apnews.com/hub/ryder-cup

United States captain Keegan Bradley reacts on the second hole at Bethpage Black golf course during the Ryder Cup golf tournament, Saturday, Sept. 27, 2025, in Farmingdale, N.Y. (AP Photo/Robert Bukaty)

Europe captain Luke Donald poses with the trophy after winning the Ryder Cup golf tournament against the United States on the Bethpage Black golf course, Sunday, Sept. 28, 2025, in Farmingdale, N.Y. (AP Photo/Lindsey Wasson)

Europe's Shane Lowry celebrates after making the winning putt putt on the 18th hole during their singles match on the Bethpage Black golf course at the Ryder Cup golf tournament, Sunday, Sept. 28, 2025, in Farmingdale, N.Y. (AP Photo/Matt Slocum)

Europe's Rory McIlroy poses with the trophy after winning the Ryder Cup golf tournament against the United States on the Bethpage Black golf course, Sunday, Sept. 28, 2025, in Farmingdale, N.Y. (AP Photo/Seth Wenig)

Europe's Shane Lowry and Justin Rose celebrate with the trophy after winning the Ryder Cup golf tournament against the United States on the Bethpage Black golf course, Sunday, Sept. 28, 2025, in Farmingdale, N.Y. (AP Photo/Robert Bukaty)
WASHINGTON (AP) — The Food and Drug Administration commissioner's effort to drastically shorten the review of drugs favored by President Donald Trump's administration is causing alarm across the agency, stoking worries that the plan may run afoul of legal, ethical and scientific standards long used to vet the safety and effectiveness of new medicines.
Marty Makary's program is causing new anxiety and confusion among staff already rocked by layoffs, buyouts and leadership upheavals, according to seven current or recently departed staffers. The people spoke to The Associated Press on the condition of anonymity because they were not authorized to discuss confidential agency matters.
At the highest levels of the FDA, questions remain about which officials have the legal authority to sign off on drugs cleared under the Commissioner’s National Priority Voucher program, which promises approval in as little as one month for medicines that support “U.S. national interests.”
Traditionally, approval decisions have nearly always been handled by FDA review scientists and their immediate supervisors, not the agency’s political appointees and senior leaders.
But drug reviewers say they've received little information about the new program's workings. And some staffers working on a highly anticipated anti-obesity pill were recently told they can skip certain regulatory steps to meet top officials' aggressive deadlines.
Outside experts point out that FDA drug reviews — which range from six to 10 months — are already the fastest in the world.
“The concept of doing a review in one to two months just does not have scientific precedent,” said Dr. Aaron Kesselheim, a professor at Harvard Medical School. “FDA cannot do the same detailed review that it does of a regular application in one to two months, and it doesn’t have the resources to do it.”
On Thursday Reuters reported that FDA officials have delayed the review of two drugs in the program, in part due to safety concerns, including the death of a patient taking one of the medications.
Health and Human Services spokesman Andrew Nixon said the voucher program prioritizes “gold standard scientific review” and aims to deliver “meaningful and effective treatments and cures."
The program remains popular at the White House, where pricing concessions announced by the Republican president have repeatedly been accompanied by FDA vouchers for drugmakers that agree to cut their prices.
For instance, when the White House announced that Eli Lilly and Novo Nordisk would reduce prices on their popular obesity drugs, FDA staffers had to scramble to vet new vouchers for both companies in time for Trump's news conference, according to multiple people involved in the process.
That’s sparked widespread concern that FDA drug reviews — long pegged to objective standards and procedures — have become open to political interference.
“It’s extraordinary to have such an opaque application process, one that is obviously susceptible to politicization,” said Paul Kim, a former FDA attorney who now works with pharmaceutical clients.
Many of the concerns around the program stem from the fact that it hasn't been laid out in federal rules and regulations.
The FDA already has more than a half-dozen programs intended to speed up or streamline reviews for promising drugs — all approved by Congress, with regulations written by agency staff.
In contrast, information about the voucher program is mostly confined to an agency website. Drugmakers can apply by submitting a 350-word “statement of interest.”
Increasingly, agency leaders such as Dr. Vinay Prasad, the FDA’s top medical officer and vaccine center director, have been contacting drugmakers directly about awarding vouchers. That’s created quandaries for FDA staffers on even basic questions, such as how to formally award a voucher to a company that didn’t request one.
Nixon, the HHS spokesman, said that voucher submissions are evaluated by “a senior, multidisciplinary review committee,” led by Prasad.
Questions about the legality of the program led the FDA’s then-drug director, Dr. George Tidmarsh, to decline to sign off on approvals under the pathway, according to several people with direct knowledge of the matter. Tidmarsh resigned from the agency in November after a lawsuit challenging his conduct on issues unrelated to the voucher program.
After his departure, Sara Brenner, the FDA’s principal deputy commissioner, was set to have the power to decide, but she also declined the role after looking further into the legal implications, according to the people. Currently the agency’s deputy chief medical officer, Dr. Mallika Mundkur, who works under Prasad, is taking on the responsibility.
Giving final approval to a drug carries significant legal risks, essentially certifying that the medicine meets FDA standards for safety and effectiveness. If unexpected safety problems later emerge, both the agency and individual staffers could be pulled into investigations or lawsuits.
Traditionally, approval comes from FDA drug office directors, made in consultation with a team of reviewers. Under the voucher program, approval comes through a committee vote by senior agency leaders led by Prasad, according to multiple people familiar with the process. Staff reviewers don't get a vote.
“It is a complete reversal from the normal review process, which is traditionally led by the scientists who are the ones immersed in the data,” said Kesselheim, who is a lawyer and a medical researcher.
Not everyone sees problems with the program. Dan Troy, the FDA’s top lawyer under President George W. Bush, a Republican, says federal law gives the commissioner broad discretion to reorganize the handling of drug reviews.
Still, he says, the voucher program, like many of Makary’s initiatives, may be short-lived because it isn't codified.
“If you live by the press release then you die by the press release,” Troy said. “Anything that they’re doing now could be wiped out in a moment by the next administration.”
Initially framed as a pilot program of no more than five drugs, it has expanded to 18 vouchers awarded, with more under consideration. That puts extra pressure on the agency’s drug center, where 20% of the staff has left through retirements, buyouts or resignations over the past year.
When Makary unveiled the program in October there were immediate concerns about the unprecedented power he would have in deciding which companies benefit.
Makary then said that nominations for drugs would come from career staffers. Indeed, some of the early drugs were recommended by FDA reviewers, according to two people familiar with the process. They said FDA staffers deliberately selected drugs that could be vetted quickly.
But, increasingly, selection decisions are led by Prasad or other senior officials, sometimes unbeknownst to FDA staff, according to three people. In one case, FDA reviewers learned from GlaxoSmithKline representatives that Prasad had contacted the company about a voucher.
Access to Makary is limited because he does not use a government email account to do business, according to people familiar with the matter, breaking with longstanding precedent.
Once a voucher is awarded, some drugmakers have their own interpretation of the review timeline — creating further confusion and anxiety among staff.
Two people involved in the ongoing review of Eli Lilly's anti-obesity pill said company executives initially told the FDA they expected the drug approved within two months.
The timeline alarmed FDA reviewers because it did not include the agency's standard 60-day prefiling period, when staffers check the application to ensure it isn’t missing essential information. That 60-day window has been in place for more than 30 years.
Lilly pushed for a quicker filing turnaround, demanding one week. Eventually the agency and the company agreed to a two-week period.
Nixon declined to comment on the specifics of Lilly's review but said FDA reviewers can “adjust timelines as needed.”
Staffers were pushed to keep the application moving forward, even though key pieces of data about the drug's chemistry appeared to be missing, according to one person involved in the process. When reviewers raised concerns about some of the gaps during an internal meeting, the person said, they were told by a senior official: “If the science is sound then you can overlook the regulations.”
Former reviewers and outside experts say that approach is the opposite of how FDA reviews should work: By following the regulations, staffers scientifically confirm the safety and effectiveness of drugs.
Skipping review steps could also carry risks for drugmakers if future FDA leaders decide a drug wasn’t properly vetted. Like other experts, Kesselheim says the program may not last beyond the current administration.
“They are fundamentally changing the application of the standards, but the underlying law remains what it is,” he said. “The hope is that one day we will return to these scientifically sound, legally sound principles.”
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education and the Robert Wood Johnson Foundation. The AP is solely responsible for all content.

FILE - The Food and Drug Administration seal is seen at the Hubert Humphrey Building Auditorium in Washington, April 22, 2025. (AP Photo/Jose Luis Magana, File)

Dr. Marty Makary, commissioner of the Food and Drug Administration, speaks during a press briefing at the White House, Wednesday, Jan. 7, 2026, in Washington. (AP Photo/Jacquelyn Martin)