SAN DIEGO--(BUSINESS WIRE)--Sep 30, 2025--
Endeavor BioMedicines (“Endeavor”), a clinical-stage biotechnology company developing medicines with the potential to deliver transformational clinical benefits to patients with life-threatening diseases, today announced that The Lancet Respiratory Medicine has published results from a Phase 2a trial evaluating the safety and efficacy of taladegib (ENV-101) in patients with idiopathic pulmonary fibrosis (IPF). Data from the Phase 2a trial were also featured in an ALERT ( A bstracts L eading to E volution in R espiratory Medicine T rials) session at the 2025 ERS Congress in Amsterdam — a special forum highlighting high-impact, practice-changing clinical trials in respiratory medicine.
Click to Gallery
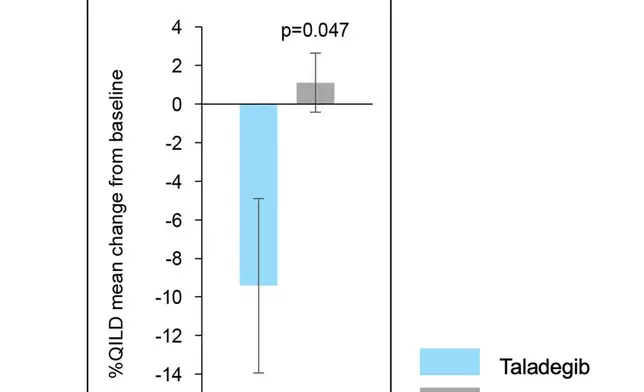
An improvement from baseline to week 12 was also seen for %QILD for the taladegib group but not for the placebo group, and there was a significant between-group difference in change from baseline to week 12 (p=0.047) with mean change from baseline of -9.4% and 1.1% in the taladegib and placebo groups, respectively.
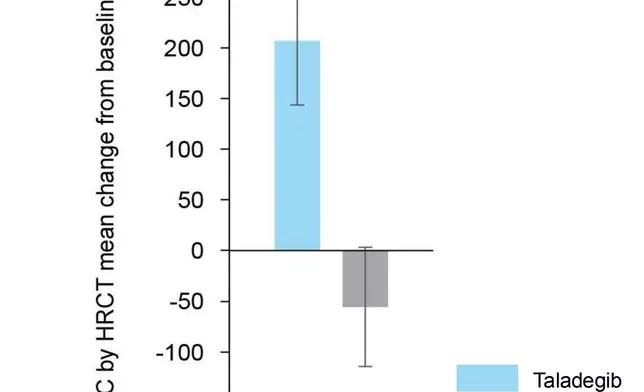
The mean change from baseline to week 12 in TLC improved significantly for the taladegib group (206.67 mL; 95% CI, 82.63 to 330.70 mL) but decreased for the placebo group (–55.58 mL; 95% CI, –170.71 to 59.55 mL) resulting in a significant between-group change from baseline to week 12 in TLC of 257.0 mL (95% CI, 86.8 to 427.2 mL; p=0.004).
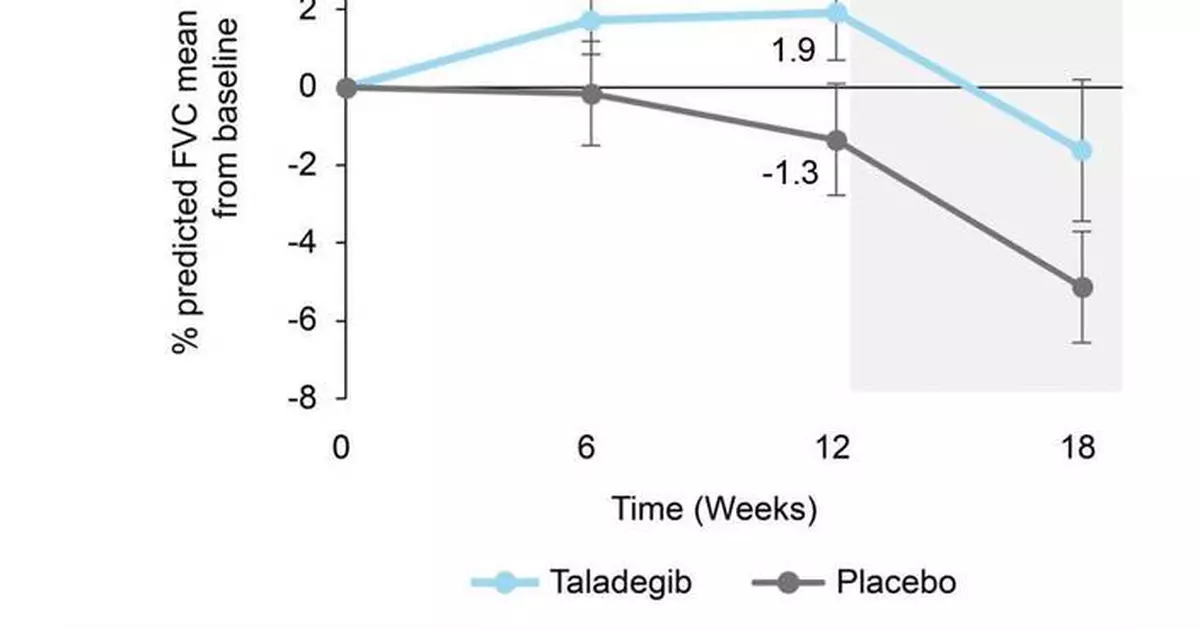
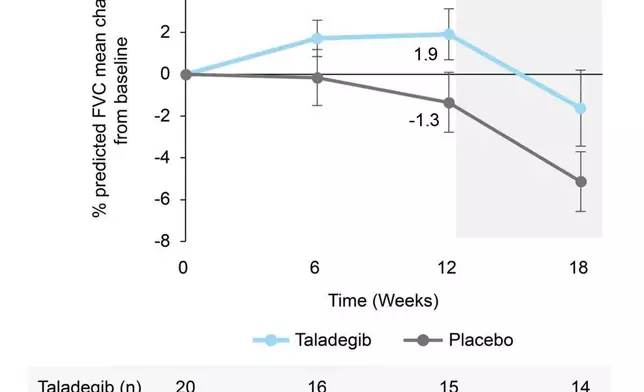
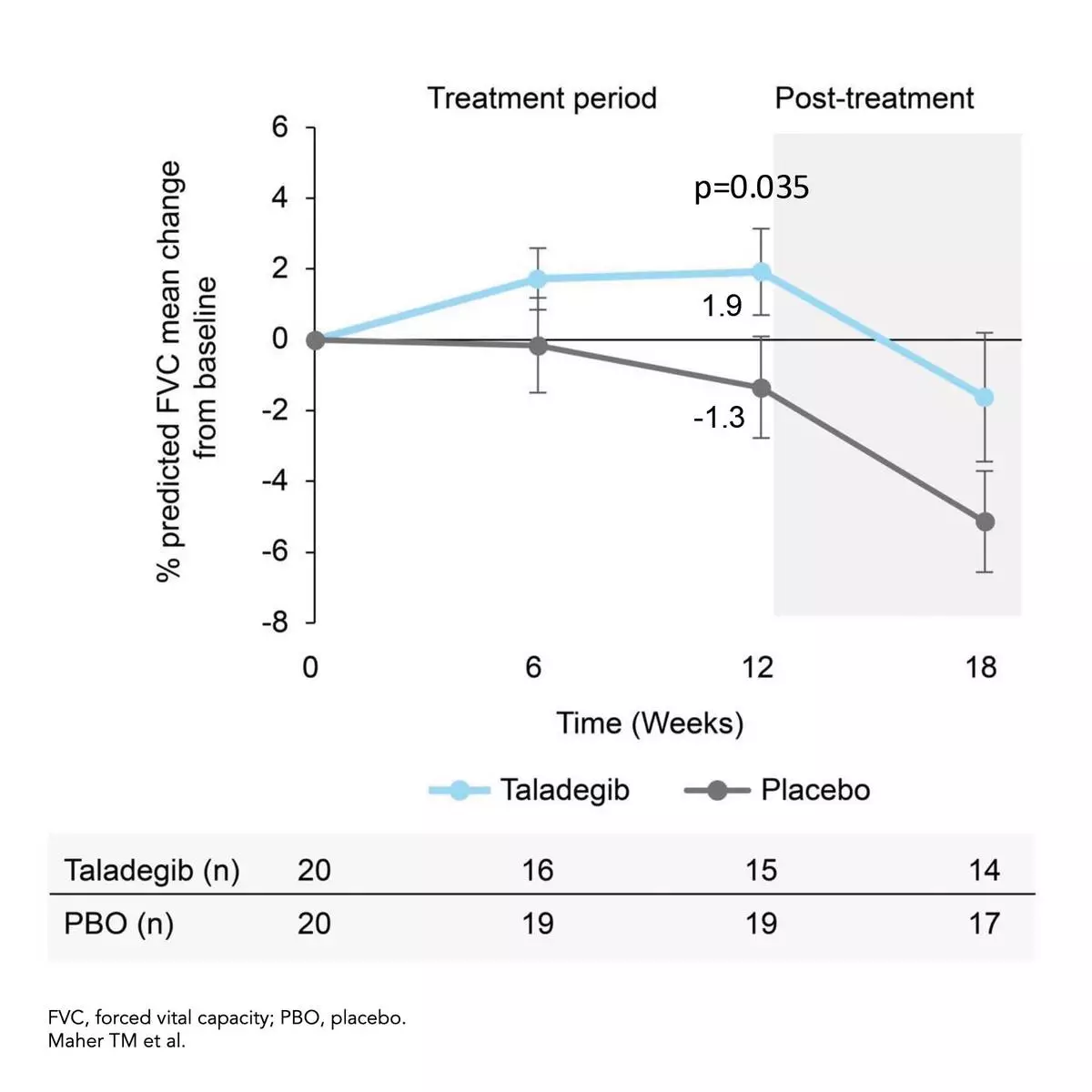
The between-group difference from baseline to week 12 in percent predicted forced vital capacity (FVC) significantly favored taladegib (3.95%; 95% confidence interval [CI], 0.31% to 7.60%; p=0.035) with a mean change from baseline of 1.9% in the taladegib arm vs -1.3% for placebo.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250929248127/en/
The proof-of-concept clinical trial demonstrated that treatment with taladegib improved lung function from baseline, increased total lung capacity and reversed key measures of lung fibrosis in patients with idiopathic pulmonary fibrosis. Taladegib also demonstrated a favorable safety and tolerability profile. There were no treatment-related serious adverse events, treatment-related ≥grade 3 adverse events, or clinically meaningful safety findings.
“Patients living with idiopathic pulmonary fibrosis face a relentless and life-altering disease that affects not only their health but every aspect of daily life,” said Toby M. Maher, M.D., Ph.D., Professor of Medicine and Director of Interstitial Lung Disease at Keck School of Medicine, University of Southern California, Los Angeles. “We are encouraged by the totality of the clinical data for taladegib, as we observed not only significant improvements from baseline in FVC measured by spirometry, but also a significant increase in total lung capacity and reductions from baseline in key measures of fibrosis as measured by CT imaging. These encouraging results, published in The Lancet Respiratory Medicine and presented today during the ERS ALERT session, represent meaningful progress toward the goal of delivering an effective treatment for patients living with IPF.”
The randomized, double-blind, placebo-controlled Phase 2a clinical trial evaluated the safety and efficacy of taladegib vs. placebo in 41 patients with confirmed IPF who were treated for 12 weeks. The trial was conducted at 16 clinical sites in Australia, Canada, Malaysia, Mexico and South Korea for patients with IPF older than 40 years of age who were not on background standard of care. Patients were randomized to 200 mg of taladegib or placebo administered once daily orally for 12 weeks with a 6-week follow-up. The primary endpoint was safety as assessed by the incidence and severity of clinical laboratory abnormalities, change from baseline in vital sign measurements, oxygen saturation, frequency and severity of adverse events, and number of hospitalizations during the study. Secondary endpoints included change from baseline in forced vital capacity (FVC), time to progression (defined as either an absolute decline of ≥10% in percent predicted FVC from baseline to week 12 or death) and dyspnea score on the UCSD SOBQ. Exploratory endpoints included measures of fibrosis assessed by high-resolution computed tomography (HRCT).
Key published findings from the taladegib Phase 2a clinical trial include:
“We are honored that the results of our Phase 2a trial have been published in The Lancet Respiratory Medicine, one of the world’s most respected and top-tier medical journals, and also selected as an ALERT presentation at ERS. This recognition is a testament to the dedication of our scientific and clinical teams, the investigators, and most importantly, the patients who participated in the trial,” said John Hood, Ph.D., Co-Founder, CEO and Chairman, Endeavor BioMedicines. “Our team remains committed to advancing taladegib with our current Phase 2b WHISTLE-PF trial, which is on track and expected to be completed in 2026.”
About Idiopathic Pulmonary Fibrosis
IPF is a chronic, progressive lung disease that affects more than 150,000 adults in the United States. Although the exact cause of IPF is unknown, various environmental factors can deliver repeated injuries to lung cells that trigger abnormal wound-healing processes and life-threatening lung scarring. IPF is a chronic disease with limited treatment options and a very poor prognosis: the average life expectancy is only three to five years after diagnosis.
About Taladegib
Endeavor BioMedicines’ investigational medicine taladegib (ENV-101) is a Hedgehog signaling pathway inhibitor. By binding to and inhibiting a key receptor in the Hedgehog pathway, taladegib eliminates the myofibroblasts that cause fibrosis. This may resolve the excessive wound-healing process seen in pulmonary fibrosis, improving lung volume and function.
About Endeavor BioMedicines
Endeavor BioMedicines is a clinical-stage biotechnology company developing medicines with the potential to deliver transformational clinical benefits to patients with life-threatening diseases. Endeavor’s lead candidate, taladegib (ENV-101), is an inhibitor of the Hedgehog signaling pathway in development for fibrotic lung diseases, including idiopathic pulmonary fibrosis (IPF). More information is available at www.endeavorbiomedicines.com and on LinkedIn or X.
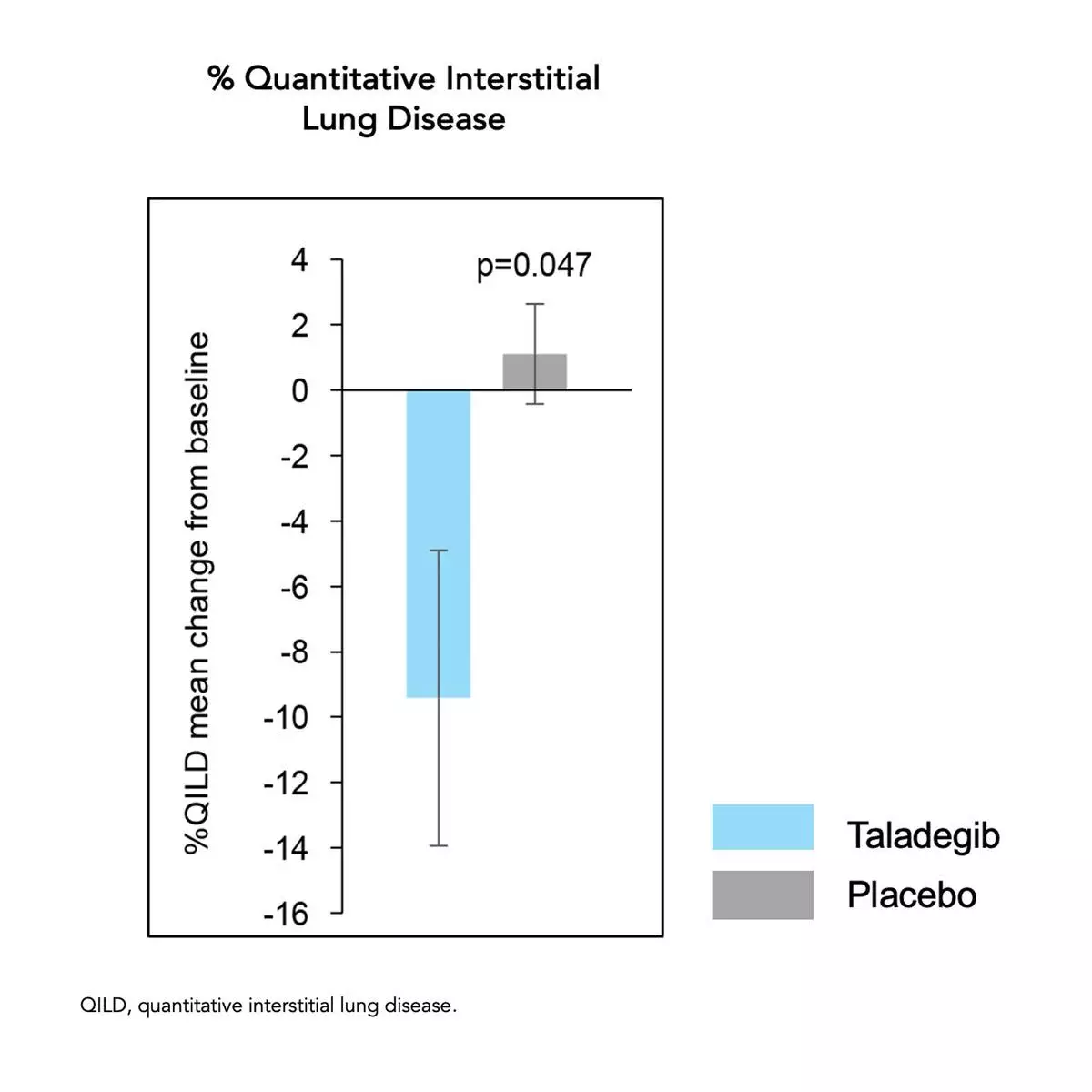
An improvement from baseline to week 12 was also seen for %QILD for the taladegib group but not for the placebo group, and there was a significant between-group difference in change from baseline to week 12 (p=0.047) with mean change from baseline of -9.4% and 1.1% in the taladegib and placebo groups, respectively.
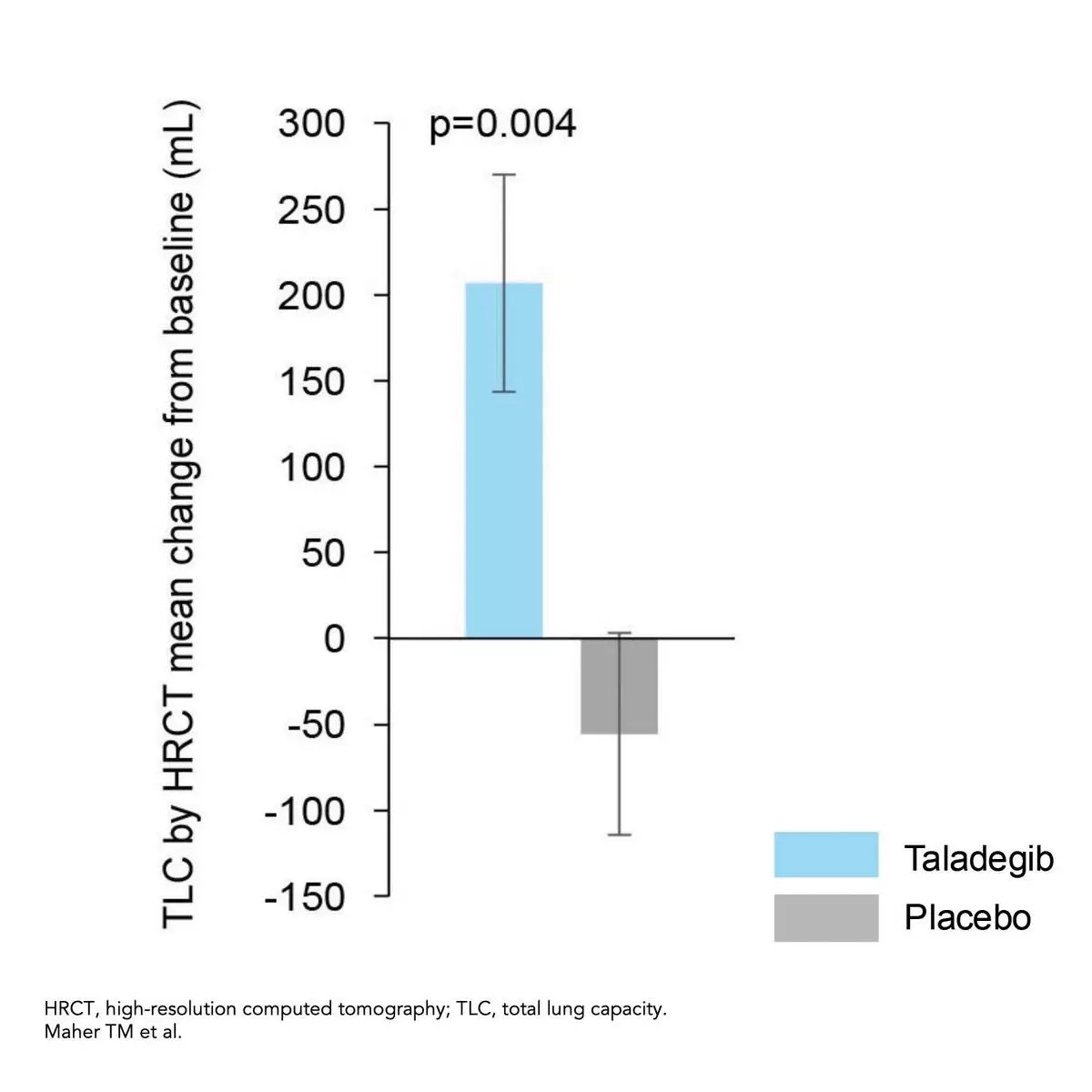
The mean change from baseline to week 12 in TLC improved significantly for the taladegib group (206.67 mL; 95% CI, 82.63 to 330.70 mL) but decreased for the placebo group (–55.58 mL; 95% CI, –170.71 to 59.55 mL) resulting in a significant between-group change from baseline to week 12 in TLC of 257.0 mL (95% CI, 86.8 to 427.2 mL; p=0.004).
![The between-group difference from baseline to week 12 in percent predicted forced vital capacity (FVC) significantly favored taladegib (3.95%; 95% confidence interval [CI], 0.31% to 7.60%; p=0.035) with a mean change from baseline of 1.9% in the taladegib arm vs -1.3% for placebo.](https://image.bastillepost.com/1200x/wp-content/uploads/global/2025/09/f2006c76a2064c31871ddfb2af900327_GRAPHIC_1.jpg.webp)
The between-group difference from baseline to week 12 in percent predicted forced vital capacity (FVC) significantly favored taladegib (3.95%; 95% confidence interval [CI], 0.31% to 7.60%; p=0.035) with a mean change from baseline of 1.9% in the taladegib arm vs -1.3% for placebo.
NEW YORK (AP) — The U.S. stock market is drifting around its all-time high on Wednesday, while the U.S. dollar’s value stabilizes against other currencies after falling to its lowest level in nearly four years.
The S&P 500 edged up by 0.1%, coming off its latest record. The Dow Jones Industrial Average was down 63 points, or 0.1%, as of 10:15 a.m. Eastern time, and the Nasdaq composite was 0.4% higher.
Some Big Tech companies helped support the market following an encouraging report from ASML. The Dutch company, whose machinery helps make chips, gave a forecast for 2026 revenue that topped analysts’ expectations.
ASML's customers have been notably more encouraged about the medium term, CEO Christophe Fouquet said, mostly because of expectations for “the sustainability” of demand related to the artificial-intelligence boom. That helped allay some concerns that the AI frenzy has gone overboard and created a potential bubble that may burst.
Nvidia, the stock that’s become the poster child of the AI boom, climbed 1.7% and was the strongest single force lifting the S&P 500. ASML’s stock that trades in the United States swung from an early gain to a drop of 1%.
Stocks elsewhere in the market were mixed following the latest flurry of profit reports.
Seagate Technology jumped 16.8% for one of the market's biggest gains after the seller of hard drives and other data-storage products reported bigger profit and revenue than analysts expected. CEO Dave Mosley cited AI applications for its strong performance, among other things.
Starbucks climbed 5.3% after its revenue for the latest quarter topped analysts’ expectations, thanks in part to a viral bear cup. That was even though its profit for the end of 2025 fell short of analysts’ targets.
Elevance Health rose 4.9% after reporting a stronger profit than analysts expected. That helped it recover some of its stock's 14.3% sell-off from the prior day, when it and other health insurers got walloped by a proposed rate increase for Medicare Advantage by the U.S. government that fell well short of what investors hoped.
But Amphenol’s stock tumbled 12.8% even though the maker of fiber-optic connectors and other high-tech equipment reported stronger growth in profit and revenue for the end of 2025 than analysts had forecast. Expectations were high for the company after its stock came into the day with an already big surge of 23% for the young year so far.
Companies across the market are under pressure to deliver solid growth in profits following the record-setting runs for their stock prices. Stock prices tend to follow the path of corporate profits over the long term, and earnings need to rise to quiet criticism that stock prices have grown too expensive.
Apple slipped 0.7% ahead of its profit report coming on Thursday, and it was one of the heaviest weights on the S&P 500.
In the foreign-currency market, the U.S. dollar found some stability and was up against the British pound, Japanese yen and others. A day earlier, an index measuring the U.S. dollar’s value against several of its peers dropped to its weakest level since early 2022.
The dollar’s value has been generally falling since President Donald Trump entered the White House last year, and its descent accelerated after Trump threatened tariffs earlier this month against several European countries that he said opposed his taking control of Greenland.
Such threats, along with worries about risks like the U.S. government’s heavy debt, have periodically pushed global investors to step away from U.S. markets, a move that’s come to be called “Sell America.”
In the bond market, Treasury yields held relatively steady ahead of an announcement coming in the afternoon from the Federal Reserve on interest rates. The widespread expectation is that it will hold its main interest rate steady.
The Fed cut rates several times last year in hopes of shoring up the job market, but inflation remains stubbornly above its 2% target. Lower interest rates could worsen inflation while giving the economy a boost.
Lower interest rates could also further undercut the U.S. dollar’s value, which would help U.S. exporters. Trump has been pushing aggressively for lower rates.
The yield on the 10-year Treasury inched up to 4.25% from 4.24% late Tuesday.
As global investors have stepped away from the U.S. dollar due to political instability and other worries, prices have surged for gold and other metals as investors searched for something safer to own. Gold’s price topped $5,000 per ounce this week for the first time, and it added another 3.5% to $5,258.70.
In stock markets abroad, indexes sank in Europe following better performances in Asia.
South Korea’s Kospi rose 1.7% to another record, thanks in part to a 5.1% leap for chip company SK Hynix, while Hong Kong’s Hang Seng rallied 2.6%.
AP Business Writers Matt Ott and Elaine Kurtenbach contributed.

The Fearless Girl statue stands in the snow in front of the New York Stock Exchange, Monday, Jan. 26, 2026. (AP Photo/Richard Drew)
Specialist Michael Pistillo works at his post on the floor of the New York Stock Exchange, Monday, Jan. 26, 2026. (AP Photo/Richard Drew)

People stand in front of an electronic stock board showing Japan's Nikkei index at a securities firm Wednesday, Jan. 28, 2026, in Tokyo. (AP Photo/Eugene Hoshiko)
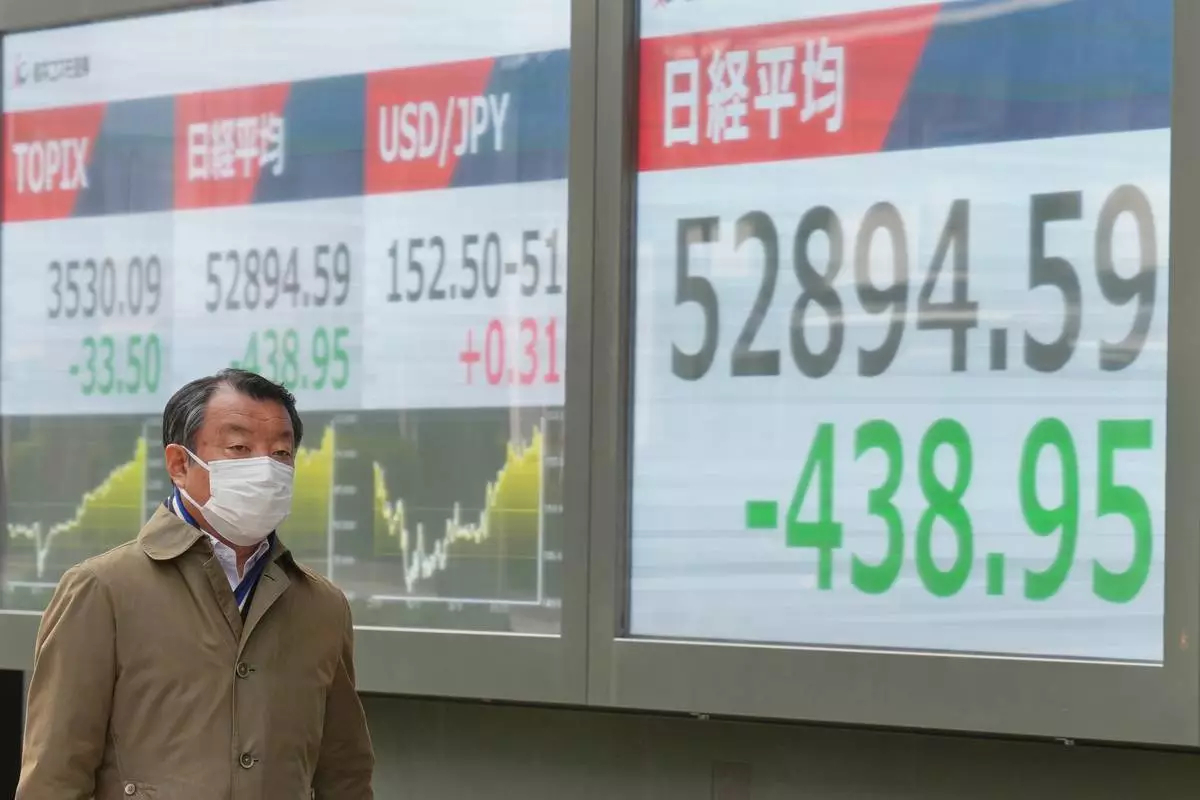
A person walks in front of an electronic stock board showing Japan's Nikkei index at a securities firm Wednesday, Jan. 28, 2026, in Tokyo. (AP Photo/Eugene Hoshiko)

A person walks in front of an electronic stock board showing Shanghai, Nikkei and New York Dow indexes at a securities firm Wednesday, Jan. 28, 2026, in Tokyo. (AP Photo/Eugene Hoshiko)

A person stands in front of an electronic stock board showing Japan's Nikkei index at a securities firm Wednesday, Jan. 28, 2026, in Tokyo. (AP Photo/Eugene Hoshiko)



![The between-group difference from baseline to week 12 in percent predicted forced vital capacity (FVC) significantly favored taladegib (3.95%; 95% confidence interval [CI], 0.31% to 7.60%; p=0.035) with a mean change from baseline of 1.9% in the taladegib arm vs -1.3% for placebo.](https://image.bastillepost.com/1200x/wp-content/uploads/global/2025/09/f2006c76a2064c31871ddfb2af900327_GRAPHIC_1.jpg.webp)