NEW HAVEN, Conn.--(BUSINESS WIRE)--Dec 11, 2025--
Veradermics, Incorporated, a dermatologist-founded, late clinical-stage biopharmaceutical company developing potentially first-in-class therapeutics for common dermatologic conditions, today announced completion of enrollment in its Phase 2/3 registration-directed clinical trial evaluating VDPHL01, the potential first-ever extended-release oral minoxidil, for the treatment of male pattern hair loss. The company’s second Phase 3 male trial is actively enrolling, marking significant progress in the development of VDPHL01.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20251211386584/en/
The Phase 2/3 male trial is a multi-center, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of VDPHL01 at two dose strengths (8.5 mg once-daily and twice-daily) over 52 weeks in 519 male participants with mild to moderate pattern hair loss. The co-primary endpoints are change in non-vellus hair count and patient-reported hair coverage benefit at 24 weeks.
Currently, the only approved oral prescription option for male pattern hair loss is finasteride, a hormonal treatment that is associated with tolerability issues, including reproductive, psychological and hormonal side effects. If approved, VDPHL01 would become the first and only non-hormonal oral treatment available for male pattern hair loss.
“Completing enrollment in this Phase 2/3 trial is an important milestone for Veradermics and for the millions of men seeking a well-tolerated, non-hormonal treatment for hair loss. Despite widespread off-label use, oral minoxidil was not intentionally designed for hair growth nor evaluated in a long-term registration-directed program. VDPHL01 is purpose-built to change that,” said Reid Waldman, M.D., Chief Executive Officer of Veradermics. “With Phase 3 trials in women and men underway, we hope to generate the evidence physicians have been asking for – data that can potentially help set a new standard in treating pattern hair loss. We believe this milestone moves us closer to our goal of delivering a new therapy that can offer convenience and improved consistency, speed, intensity and tolerability.”
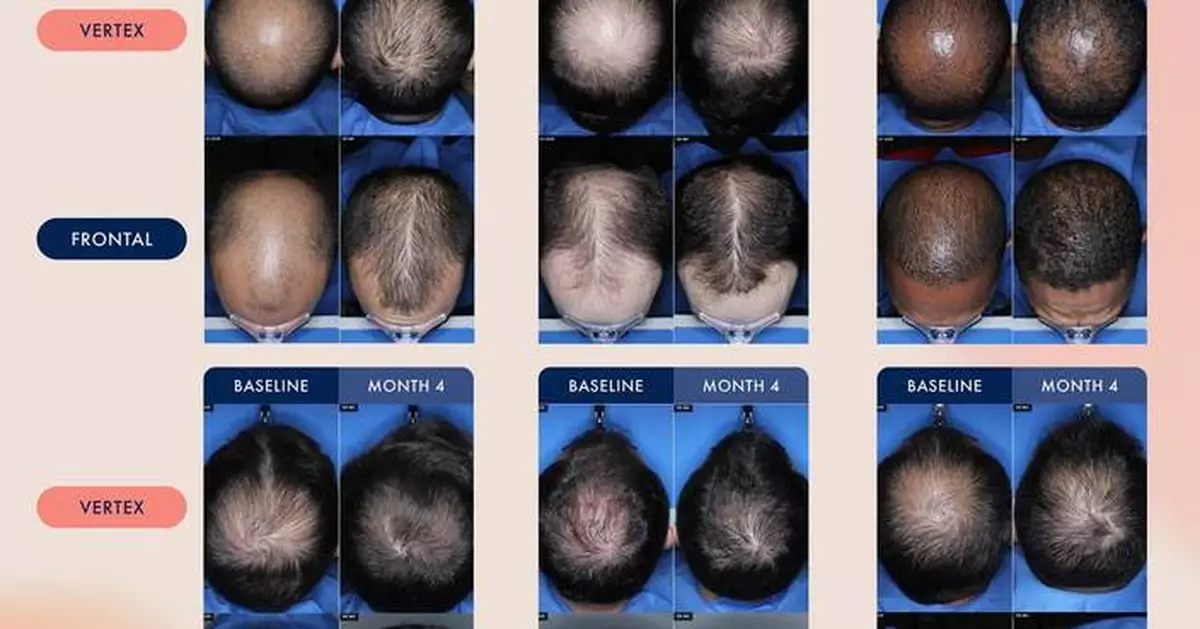
VDPHL01’s novel extended-release formulation is designed to extend exposure of minoxidil to hair follicles over time. This release profile is intended to enable fast, consistent and intense hair growth, while avoiding concentration spikes above minoxidil’s identified cardiac activity threshold, the blood levels at which cardiac effects are first observed. The company recently shared preliminary data from the male cohort in its ongoing Phase 2 trial of VDPHL01, including:
Full data from the Phase 2 trials evaluating VDPHL01 in both women and men with pattern hair loss is anticipated in 2026.
Phase 3 Trials Underway for Women and Men with Pattern Hair Loss
Veradermics is actively recruiting participants for its second Phase 3 trial in males (NCT06972264) and its Phase 2/3 trial in females (NCT07146022) with pattern hair loss. For more information about trial enrollment, please visit www.phlstudy.com or www.phlstudy.com/female.
About Pattern Hair Loss
Pattern hair loss, also known as androgenetic alopecia, affects an estimated 80 million people in the United States (30 million women and 50 million men). Pattern hair loss can have a significant impact on quality of life, affecting an individual’s mental health and relationships. People with pattern hair loss often experience depression, low self-esteem and social withdrawal. There have been no new FDA-approved prescription medicines for pattern hair loss in nearly 30 years. In addition to prescription medicines, current treatments include over-the-counter “nutraceuticals” that produce inconsistent results and contribute to high dissatisfaction among patients and healthcare providers. The prevalence of pattern hair loss and the market demand for new treatments contribute to making this the largest aesthetics market worldwide, projected to reach approximately $30 billion by 2028.
About VDPHL01
VDPHL01 (extended-release minoxidil tablet) is an investigational, orally available non-hormonal drug in Phase 3 development for pattern hair loss in both women and men. VDPHL01 leverages extended-release technology to deliver a minoxidil product with the potential for improved efficacy and safety. The proprietary extended-release formulation utilizes a gel matrix designed to deliver long-lasting, steady release of minoxidil for sustained absorption. VDPHL01 has been shown to avoid the high peak concentrations of immediate-release oral minoxidil, while extending time above the minimum hair growth threshold to increase time for hair to grow. We believe VDPHL01 is currently the only non-hormonal oral treatment in clinical development for hair loss in both women and men.
About Veradermics
Veradermics is a dermatologist-founded, late clinical-stage biopharmaceutical company focused on turning everyday dermatology and aesthetics problems into clear, proven care. Veradermics’ lead program, VDPHL01, is an extended-release oral minoxidil tablet in Phase 3 development for the treatment of pattern hair loss — the largest aesthetics condition worldwide, affecting both women and men, with no new FDA-approved prescription therapies in nearly 30 years. In addition, Veradermics is advancing a pipeline of potentially differentiated product candidates designed to address high-value dermatologic conditions with little to no proven solutions, combining proven mechanisms with innovative formulations that are designed to optimize efficacy, safety and patient convenience. For additional information, visit www.veradermics.com and follow us on LinkedIn and Instagram.
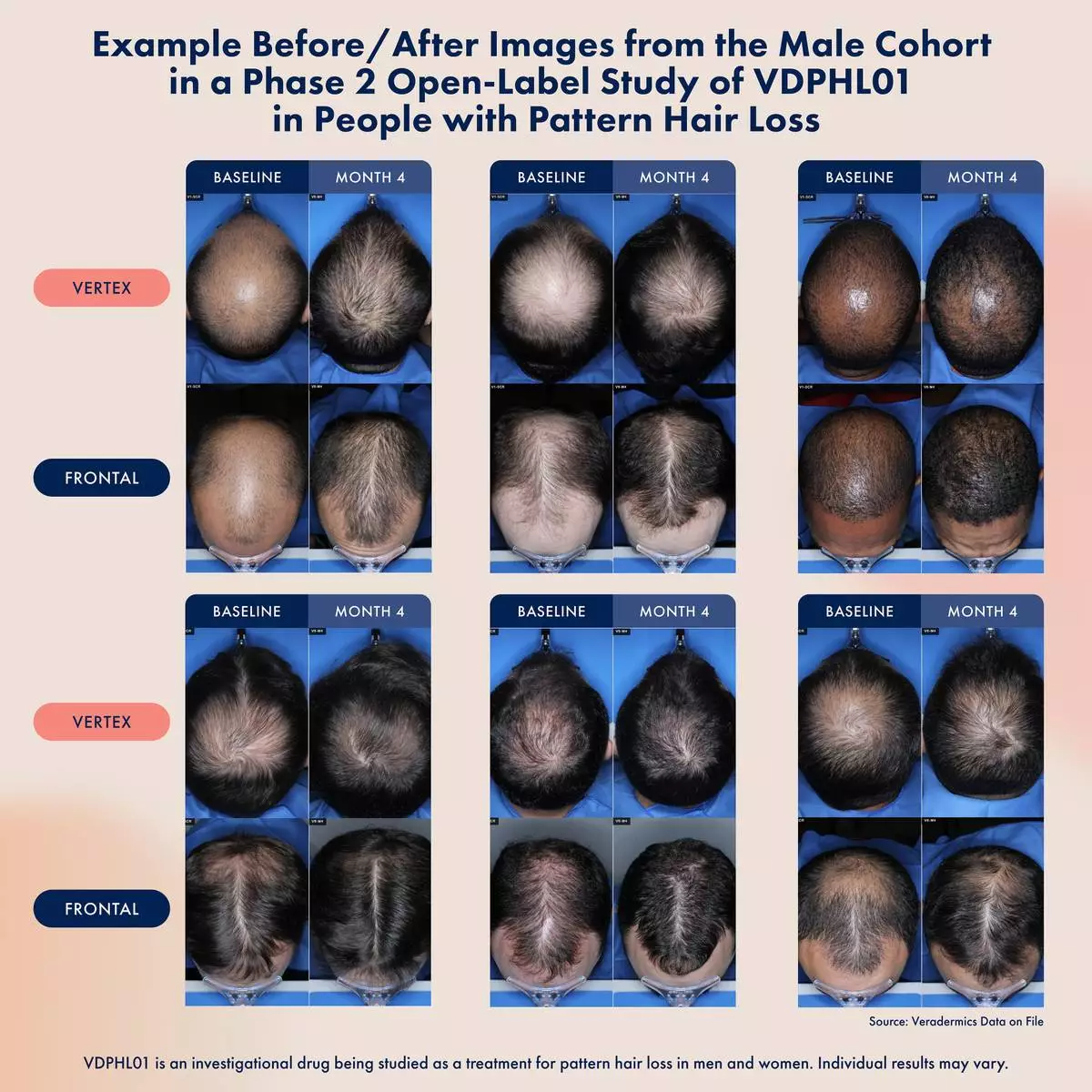
Image of subjects from preliminary Phase 2 readout shared by Veradermics in October