ATLANTA--(BUSINESS WIRE)--Dec 15, 2025--
Nephrodite, Inc., a medical device company developing an implantable, continuous renal replacement system, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to the company’s Holly™ implantable, continuous dialysis system.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20251215001657/en/
The designation recognizes Holly as a novel, transformative therapy addressing end-stage kidney disease (ESKD) which affects more than 850,000 people in the U.S., costing more than $50 billion annually. Current treatment options including hemodialysis and peritoneal dialysis limit mobility and patients’ quality of life, with no adequately effective alternatives available to patients since the advent of hemodialysis more than 70 years ago.
“I’m proud of our stellar team for earning this extraordinary recognition. The designation reinforces the scientific and clinical significance of what we’re building,” said Nikhil L. Shah, DO, MPH, Co-Founder and CEO of Nephrodite. “Holly was designed from the ground up to free patients from the cycle of center-based dialysis. Breakthrough status enables close collaboration with regulators and accelerates our path toward first-in-human studies.”
Data from Nephrodite’s successful multi-day large animal study demonstrating sustained kidney function replacement with strong safety and performance outcomes helped enable the FDA’s Breakthrough Device Designation. The FDA Breakthrough Device Designation is reserved for technologies that offer more effective treatment for life-threatening conditions and designed to expedite patient access to promising innovations. The designation allows Nephrodite to benefit from expedited review and enhanced FDA guidance.
“Dialysis sustains life, but at tremendous cost to a patient’s freedom and physiology,” said Hiep T. Nguyen, MD, Co-Founder and SVP of Science and Technology at Nephrodite. “Holly represents a complete rethinking of kidney replacement, with a continuously functioning implant capable of matching the body’s natural rhythm. It’s both a scientific milestone and a human one.”
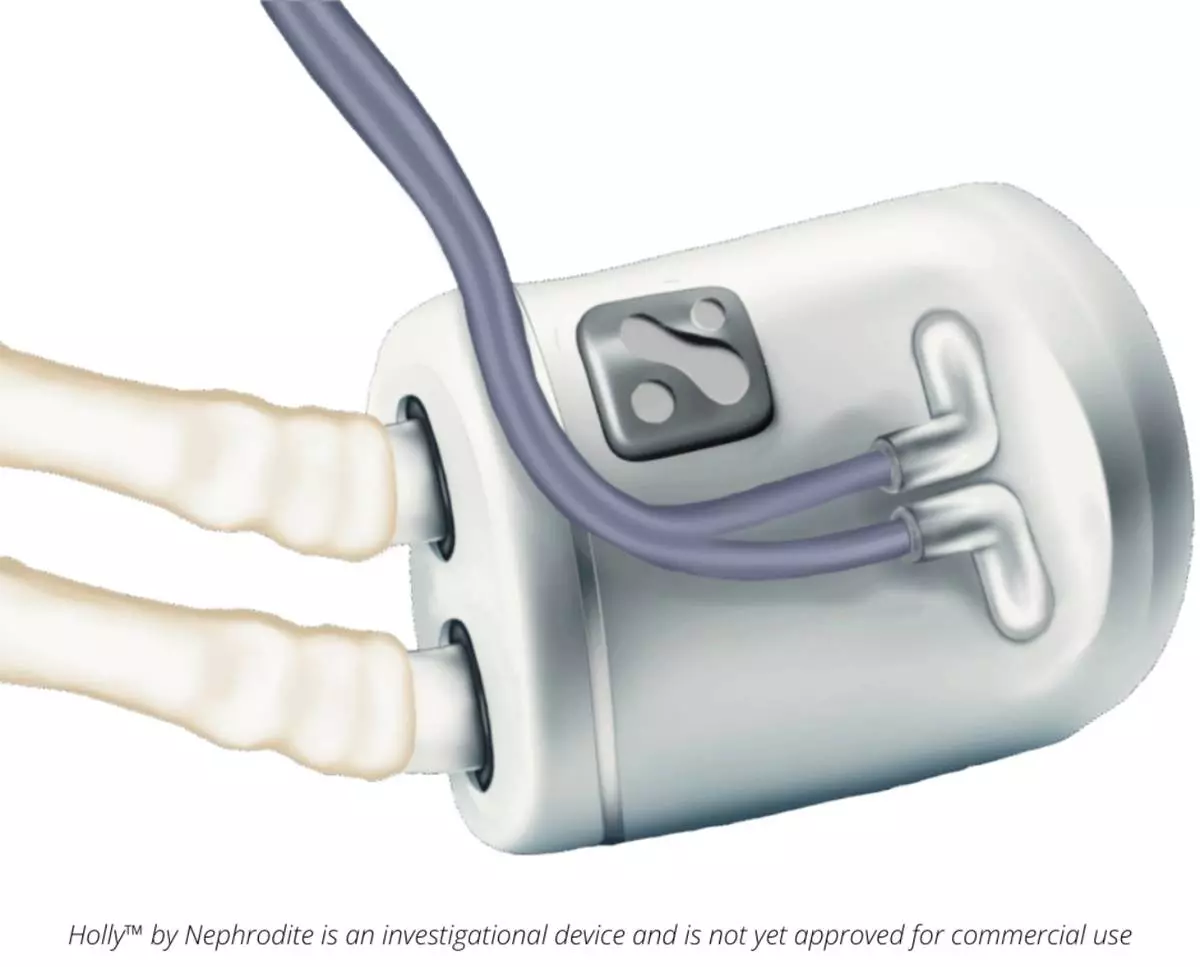
The Holly system is a first-of-its-kind implantable platform engineered to function continuously inside the body, replacing natural kidney function. The proprietary platform combines advanced hemofiltration technology with biocompatible materials optimized for long-term implantation and physiologic performance. The device is designed to continuously filter waste, balance fluids, and manage electrolytes without the need for frequent clinic visits required by traditional, center-based dialysis.
The Holly system integrates intelligent sensors, machine learning algorithms, and secure remote monitoring for physician oversight and individualized therapy. The internal implant is being designed to link through a simple external interface to a small, portable home unit used nightly for dialysis support.
Building on the Breakthrough Device designation and the preclinical animal study findings, Nephrodite is preparing for Good Laboratory Practice (GLP) studies and subsequent regulatory submissions to enable first-in-human clinical trials.
Follow Nephrodite on LinkedIn and visit nephrodite.com for ongoing updates.
Holly™ is an investigational device and is not yet approved for commercial use.
About Nephrodite
Nephrodite is a medical device company dedicated to creating a better alternative to dialysis by developing Holly, an implantable, continuous renal replacement device designed to restore freedom, function and independence in people living with end-stage kidney disease. Founded by board-certified urologists Nikhil L. Shah, DO, MPH, and Hiep T. Nguyen, MD, the company combines deep clinical expertise with engineering innovation to deliver a true renal replacement platform. Headquartered in Atlanta, Georgia, with operations in Mumbai, India, Nephrodite is backed by leading investors and supported by a robust intellectual property portfolio.
Nephrodite Earns FDA’s First Breakthrough Device Designation for an Implantable Kidney Replacement System










