|
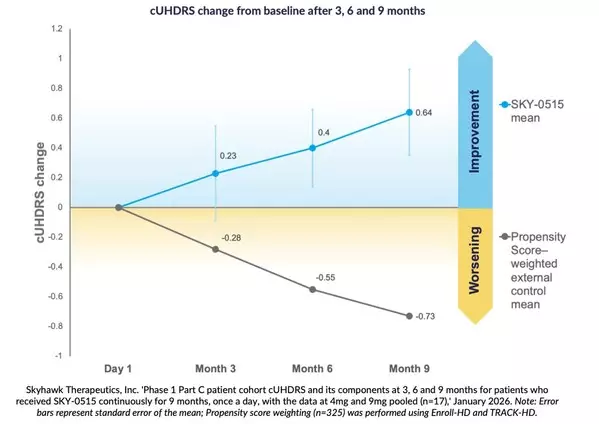
Nine-month findings show mean improvement in Composite Unified Huntington's Disease Rating Scale from baseline of +0.64 points, compared to natural history expected worsening of cUHDRS in symptomatic patients of -0.73 points over nine months, based on propensity score weighting.
Skyhawk also announces SKY-0515's Phase 2/3 FALCON-HD trial has expanded worldwide. Skyhawk has now dosed more than 90 patients.
BOSTON, Jan. 27, 2026 /PRNewswire/ -- Skyhawk Therapeutics, Inc., a clinical-stage biotechnology company developing novel small molecule therapies to modulate critical RNA targets, today announces positive results from the nine month interim analysis of the Company's investigational treatment for Huntington's disease (HD) with SKY-0515.
Treatment with SKY-0515 results in dose-dependent reductions of mHTT protein in blood of 62% at the 9mg dose, and dose-dependent PMS1 mRNA reduction of 26%. PMS1 is a key driver of somatic CAG repeat expansion and HD pathology. SKY-0515 has also demonstrated excellent central nervous system exposure and been generally safe and well tolerated.
At three, six and nine months, patients receiving SKY-0515 in the Part C patient cohort of the Phase 1 clinical trial of SKY-0515, demonstrate mean Composite Unified Huntington's Disease Rating Scale (cUHDRS) improvement from baseline. At nine months, in a pooled analysis, this improvement is +0.64 points compared to expected worsening at nine months of cUHDRS in symptomatic patients of -0.73 points, based on propensity score weighting using Enroll-HD and TRACK-HD.
"I am very encouraged by these safety and early efficacy data from SKY-0515's Phase 1 Part C trial in patients, showing divergence in cUHDRS away from expected natural history deterioration at the three, six, and nine month prespecified analyses," said Ed Wild, Professor of Neurology at University College London. "SKY-0515 continues to reduce mHTT protein to the greatest extent demonstrated by any therapeutic tested to date in patients, with clinical and biomarker data showing the drug is well tolerated at all doses tested. SKY-0515's ability to reduce both mHTT and PMS1 offers a potent combination for treating Huntington's disease via two of its core pathogenic mechanisms. These open-label trial results, due to be validated in the ongoing placebo-controlled FALCON-HD trial, give an expectation of meaningful impact for people living with HD across the world – for whom an orally administered huntingtin-lowering treatment such as SKY-0515 will be truly transformative."
"Our goal for our Phase 1 study was to establish safety and biomarker activity," said Sergey Paushkin, Head of R&D at Skyhawk Therapeutics, "and the continued strength of SKY-0515's biomarker response in our nine month interim data analysis – and the improvement in the potential endpoint, cUHDRS, compared to a worsening of the cUHDRS score in the natural history data for patients - underscores SKY-0515's potential as a best in class disease-modifying therapy for HD. These interim data represent an important milestone for SKY-0515 and highlight the power of Skyhawk's platform to deliver first-in-class small molecules for devastating diseases with no approved disease-modifying therapies."
Huntington's disease is a rare, hereditary, and ultimately fatal neurodegenerative disorder that affects over 40,000 symptomatic patients in the United States, with hundreds of thousands estimated to be affected worldwide. There are currently no approved treatments which slow or halt disease progression. SKY-0515 is an orally-administered, investigational small molecule RNA modulator developed through the company's novel RNA-modulating platform, SKYSTAR®. SKY-0515 therapeutically reduces both HTT protein and PMS1 protein. PMS1 is an additional key driver of somatic CAG repeat expansion and HD pathology and should complement the benefits of reducing mutant HTT.
Skyhawk also announces today that its SKY-0515 Phase 2/3 FALCON-HD trial, open at twelve sites in Australia and New Zealand, has expanded worldwide. Skyhawk has now dosed more than 90 patients with SKY-0515.
SKY-0515 is the first Skyhawk drug in clinical trials.
Skyhawk expects to put additional small molecule drugs to treat rare neurological diseases with no approved disease modifying therapies in the clinic by the end of 2027.
About SKY-0515's Phase 1 Clinical Study
SKY-0515's Phase 1 clinical trial is a first-in-human trial designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SKY-0515 in healthy volunteers and individuals with early-stage Huntington's disease (HD). The trial is separated into three parts. Parts A and B evaluated SKY-0515 in Healthy Volunteers. Part C is a double-blind placebo-controlled parallel design study of two dose levels of SKY-0515 and placebo in individuals with early-stage HD (HD-ISS Stage 1, 2, or mild Stage 3) for 84 days followed by a 12 month extension of active treatment where all participants will receive either a low or high dose of SKY-0515 in a blinded fashion. The objectives of the study include evaluating mutant HTT protein and PMS1 mRNA. The first patients were dosed in SKY-0515's Part C in January 2025. Enrollment in Phase 1C of the SKY-0515 trial is now complete.
About SKY-0515's Phase 2/3 FALCON-HD Clinical Study
FALCON-HD (NCT06873334) is a Phase 2/3 randomized, double-blind, placebo-controlled, dose ranging study to evaluate the pharmacodynamics, safety, and efficacy of SKY-0515 in 120 participants with Stage 2 and early Stage 3 HD across 12 sites in Australia and New Zealand, and 400 participants with Stage 2 and early Stage 3 HD in 40+ worldwide sites. Eligible patients will receive a once-daily oral dose of SKY-0515 at one of three dose levels or placebo, for a treatment period of at least 12 months. The trial aims to assess the potential of SKY-0515 to modulate RNA splicing and reduce mHTT and PMS1 proteins, which are implicated in the pathology of Huntington's disease. Additional information about FALCON-HD, including participating sites and eligibility criteria, can be found at ClinicalTrials.gov and www.FALCON-HD.com.
About Skyhawk Therapeutics
Skyhawk Therapeutics is a clinical-stage biotechnology company which uses its proprietary platform, SKYSTAR®, to discover and develop small molecule RNA modulating therapies for the world's most intractable diseases. For more information visit www.skyhawktx.com.
Skyhawk Contact
Maura McCarthy
Head of Corporate Development
maura@skyhawktx.com
Nine-month findings show mean improvement in Composite Unified Huntington's Disease Rating Scale from baseline of +0.64 points, compared to natural history expected worsening of cUHDRS in symptomatic patients of -0.73 points over nine months, based on propensity score weighting.
Skyhawk also announces SKY-0515's Phase 2/3 FALCON-HD trial has expanded worldwide. Skyhawk has now dosed more than 90 patients.
BOSTON, Jan. 27, 2026 /PRNewswire/ -- Skyhawk Therapeutics, Inc., a clinical-stage biotechnology company developing novel small molecule therapies to modulate critical RNA targets, today announces positive results from the nine month interim analysis of the Company's investigational treatment for Huntington's disease (HD) with SKY-0515.
Treatment with SKY-0515 results in dose-dependent reductions of mHTT protein in blood of 62% at the 9mg dose, and dose-dependent PMS1 mRNA reduction of 26%. PMS1 is a key driver of somatic CAG repeat expansion and HD pathology. SKY-0515 has also demonstrated excellent central nervous system exposure and been generally safe and well tolerated.
At three, six and nine months, patients receiving SKY-0515 in the Part C patient cohort of the Phase 1 clinical trial of SKY-0515, demonstrate mean Composite Unified Huntington's Disease Rating Scale (cUHDRS) improvement from baseline. At nine months, in a pooled analysis, this improvement is +0.64 points compared to expected worsening at nine months of cUHDRS in symptomatic patients of -0.73 points, based on propensity score weighting using Enroll-HD and TRACK-HD.
"I am very encouraged by these safety and early efficacy data from SKY-0515's Phase 1 Part C trial in patients, showing divergence in cUHDRS away from expected natural history deterioration at the three, six, and nine month prespecified analyses," said Ed Wild, Professor of Neurology at University College London. "SKY-0515 continues to reduce mHTT protein to the greatest extent demonstrated by any therapeutic tested to date in patients, with clinical and biomarker data showing the drug is well tolerated at all doses tested. SKY-0515's ability to reduce both mHTT and PMS1 offers a potent combination for treating Huntington's disease via two of its core pathogenic mechanisms. These open-label trial results, due to be validated in the ongoing placebo-controlled FALCON-HD trial, give an expectation of meaningful impact for people living with HD across the world – for whom an orally administered huntingtin-lowering treatment such as SKY-0515 will be truly transformative."
"Our goal for our Phase 1 study was to establish safety and biomarker activity," said Sergey Paushkin, Head of R&D at Skyhawk Therapeutics, "and the continued strength of SKY-0515's biomarker response in our nine month interim data analysis – and the improvement in the potential endpoint, cUHDRS, compared to a worsening of the cUHDRS score in the natural history data for patients - underscores SKY-0515's potential as a best in class disease-modifying therapy for HD. These interim data represent an important milestone for SKY-0515 and highlight the power of Skyhawk's platform to deliver first-in-class small molecules for devastating diseases with no approved disease-modifying therapies."
Huntington's disease is a rare, hereditary, and ultimately fatal neurodegenerative disorder that affects over 40,000 symptomatic patients in the United States, with hundreds of thousands estimated to be affected worldwide. There are currently no approved treatments which slow or halt disease progression. SKY-0515 is an orally-administered, investigational small molecule RNA modulator developed through the company's novel RNA-modulating platform, SKYSTAR®. SKY-0515 therapeutically reduces both HTT protein and PMS1 protein. PMS1 is an additional key driver of somatic CAG repeat expansion and HD pathology and should complement the benefits of reducing mutant HTT.
Skyhawk also announces today that its SKY-0515 Phase 2/3 FALCON-HD trial, open at twelve sites in Australia and New Zealand, has expanded worldwide. Skyhawk has now dosed more than 90 patients with SKY-0515.
SKY-0515 is the first Skyhawk drug in clinical trials.
Skyhawk expects to put additional small molecule drugs to treat rare neurological diseases with no approved disease modifying therapies in the clinic by the end of 2027.
About SKY-0515's Phase 1 Clinical Study
SKY-0515's Phase 1 clinical trial is a first-in-human trial designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SKY-0515 in healthy volunteers and individuals with early-stage Huntington's disease (HD). The trial is separated into three parts. Parts A and B evaluated SKY-0515 in Healthy Volunteers. Part C is a double-blind placebo-controlled parallel design study of two dose levels of SKY-0515 and placebo in individuals with early-stage HD (HD-ISS Stage 1, 2, or mild Stage 3) for 84 days followed by a 12 month extension of active treatment where all participants will receive either a low or high dose of SKY-0515 in a blinded fashion. The objectives of the study include evaluating mutant HTT protein and PMS1 mRNA. The first patients were dosed in SKY-0515's Part C in January 2025. Enrollment in Phase 1C of the SKY-0515 trial is now complete.
About SKY-0515's Phase 2/3 FALCON-HD Clinical Study
FALCON-HD (NCT06873334) is a Phase 2/3 randomized, double-blind, placebo-controlled, dose ranging study to evaluate the pharmacodynamics, safety, and efficacy of SKY-0515 in 120 participants with Stage 2 and early Stage 3 HD across 12 sites in Australia and New Zealand, and 400 participants with Stage 2 and early Stage 3 HD in 40+ worldwide sites. Eligible patients will receive a once-daily oral dose of SKY-0515 at one of three dose levels or placebo, for a treatment period of at least 12 months. The trial aims to assess the potential of SKY-0515 to modulate RNA splicing and reduce mHTT and PMS1 proteins, which are implicated in the pathology of Huntington's disease. Additional information about FALCON-HD, including participating sites and eligibility criteria, can be found at ClinicalTrials.gov and www.FALCON-HD.com.
About Skyhawk Therapeutics
Skyhawk Therapeutics is a clinical-stage biotechnology company which uses its proprietary platform, SKYSTAR®, to discover and develop small molecule RNA modulating therapies for the world's most intractable diseases. For more information visit www.skyhawktx.com.
Skyhawk Contact
Maura McCarthy
Head of Corporate Development
maura@skyhawktx.com
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Skyhawk Therapeutics Announces Nine Month Interim Results in Patients from its Phase 1 Clinical Trial of SKY-0515 as a Treatment for Huntington's Disease
TOKYO, Jan. 28, 2026 /PRNewswire/ -- Club Tourism is a travel company with an extensive portfolio of outdoor experience tours across Japan, including hiking, mountaineering, walking and cycling.
Boasting one of Japan's top-tier ranges of outdoor tours, from casual nature walks perfect for first-time outdoor enthusiasts to professional mountaineering expeditions, the company offers a diverse array of itineraries departing from locations all over Japan.
Japan's abundant natural beauty — encompassing mountains, valleys and highlands — lies just beyond its urban centers.
With ever-changing seasonal landscapes, crisp fresh air and serene forest trails, stepping foot on these lands in person, rather than just browsing guidebooks, deepens the richness of any Japan travel experience immeasurably.
In recent years, there has been a growing demand among international visitors to Japan for more than just urban sightseeing; travelers increasingly seek to immerse themselves in the country's natural landscapes.
However, many are hesitant to arrange such trips independently due to concerns like not knowing how to reach trailheads, the perceived hassle of preparing equipment, and lack of confidence in their physical stamina.
In response to these needs, Club Tourism has launched a dedicated 2026 Outdoor Tour Special Page, designed with a strong focus on accessibility and reliable support systems.
Special Page URL: https://www.club-t.com/zh-CHT/sp/special/inbound/yokoso/hiking/?waad=&argument=UCwNomcJ&dmai=a697177f66f43e&utm_source=prwire&utm_medium=owned&utm_campaign=05_HR_PRinbaundo__adgc__HK2601PRriri-su_haikingutua-_prwire_owned_no&utm_term=&utm_content=
Key Highlights — Japan Inbound × Outdoor Experience
This Special Page features an extensive selection of tours, including not only professional mountaineering trips but also relaxed walking, hiking and cycling itineraries, suitable for first-time outdoor adventurers. Also, there are unique tours combining natural experiences with special events like summer festivals or firework displays.
- Station gathering & departure – eliminate travel worries: Meeting points are set at Shinkansen stops, major JR stations and stations with easy airport access. This removes the stress of carrying large luggage and the trouble of researching complex transit connections, enabling seamless access to Japan's natural landscapes.
- Guided tours & pre-planned itineraries – total peace of mind: Accompanied by experienced tour guides and with itineraries crafted with sensible time allocation, travelers don't need to worry about language barriers or safety issues. Just fully immerse yourselves in the stunning scenery.
- Diverse range for all skill levels: Tours cater to every level of physical ability and interest, from beginner-friendly routes along flat paths to advanced expeditions such as Mount Fuji climbing — the ultimate challenge to Japan's highest peak.
Highly Accessible: Featured Outdoor Tour Destinations
Mount Fuji is an icon of Japan, a diverse range of Fuji tours are available — from leisurely hikes to admire its majestic vistas, to challenging expeditions aiming for its summit.
Meeting points are flexible, with departures available from Mishima Station (a Shinkansen stop) and other conveniently located JR stations in city centers. Our guided tour service takes care of all equipment and route planning, ensuring you create unforgettable travel memories.
A premier scenic destination in Japan, home to vast marshes, crystal-clear streams and breathtaking views of the Northern Japanese Alps.
Tours depart from major stations such as Matsumoto Station, providing direct access to areas which is difficult to reach independently. With well-maintained boardwalks and numerous flat routes, visitors can enjoy a leisurely 3-hour stroll, making this destination perfect for first-time outdoor hikers and family groups alike.
*Matsumoto Station is easily accessible from Shinjuku and Nagoya. Hiking and highland trekking tours in the Hakuba Area, also departing from Matsumoto Station, come highly recommended.
A historic pilgrimage route inscribed as a UNESCO World Heritage Site.
Exploring this ancient trail independently can be challenging due to access and route selection, but Club Tourism's guided tours include bus transportation and expert guides for a worry-free experience. This is a unique opportunity to deeply connect with both Japan's natural beauty and its rich cultural heritage.
A world-famous route beloved by cyclists worldwide, connecting the islands of the Seto Inland Sea via a series of stunning bridges.
Itineraries depart from stations such as Fukuyama Station and Shin-Osaka Station, with easy access via Shinkansen and other transit. Travelers can choose between walking or cycling, allowing them to experience the refreshing sea breezes of the Seto Inland Sea at their own pace.
*Products featured on the Special Page are subject to display changes based on season, departure location and other factors.
New outdoor tours from across Japan will be added to the page in succession as they become available — stay tuned for more!
Limited-Time Campaign Now On!
Book specified tours now to enjoy exclusive discount coupons as part of our limited-time campaign:
- ¥1,000 off on bookings of ¥6,000 or more
- ¥2,000 off on bookings of ¥20,000 or more
Coupon Redemption URL: https://www.club-t.com/zh-CHT/sp/campaign/inbound/yokoso/
Ideal for These Travelers
- Seeking to complement Japan urban sightseeing with unique extra experiences
- Families and couples looking to connect with nature at a comfortable pace
- Travelers intrigued by the idea of walking through stunning scenery but deterred by the hassle of mountaineering preparations
- Anyone wanting to enjoy a hassle-free, efficient trip without worries about transportation or scheduling
About Club Tourism
As a travel company under the Kintetsu Group, Club Tourism boasts over 40 years of industry experience and has been welcoming international travelers from around the world since 2008.
Specializing in outdoor experience tours in Japan — including hiking, mountaineering, walking and cycling — the company's key strengths lie in its extensive tour range catering to all levels, from first-time adventurers to seasoned outdoor enthusiasts, and its diverse itineraries departing from locations across the country.
With a wide variety of tour types, from day trips to overnight stays, and from traditional festival experiences to hidden gem explorations, travelers can fully immerse themselves in Japan's renowned hospitality and unique culture. All itineraries are thoughtfully designed to be accessible for middle and senior travelers, ensuring a worry-free experience for all.
"On a once-in-a-lifetime trip to Japan, I want to create unforgettable memories."
Club Tourism's tours are crafted to answer this very wish.
Company Profile
Company Name: Club Tourism Co., Ltd.
Address: NBF Toyosu Canal Front, 5-6-52 Toyosu, Koto-ku, Tokyo, Japan
Business: Planning and operation of domestic and international package tours, theme-based travel
Official Website: https://www.club-t.com/
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

2026 Japan Travel with Club Tourism: Gather at Shinkansen & Major JR stations, direct to Japan's natural wonders

2026 Japan Travel with Club Tourism: Gather at Shinkansen & Major JR stations, direct to Japan's natural wonders