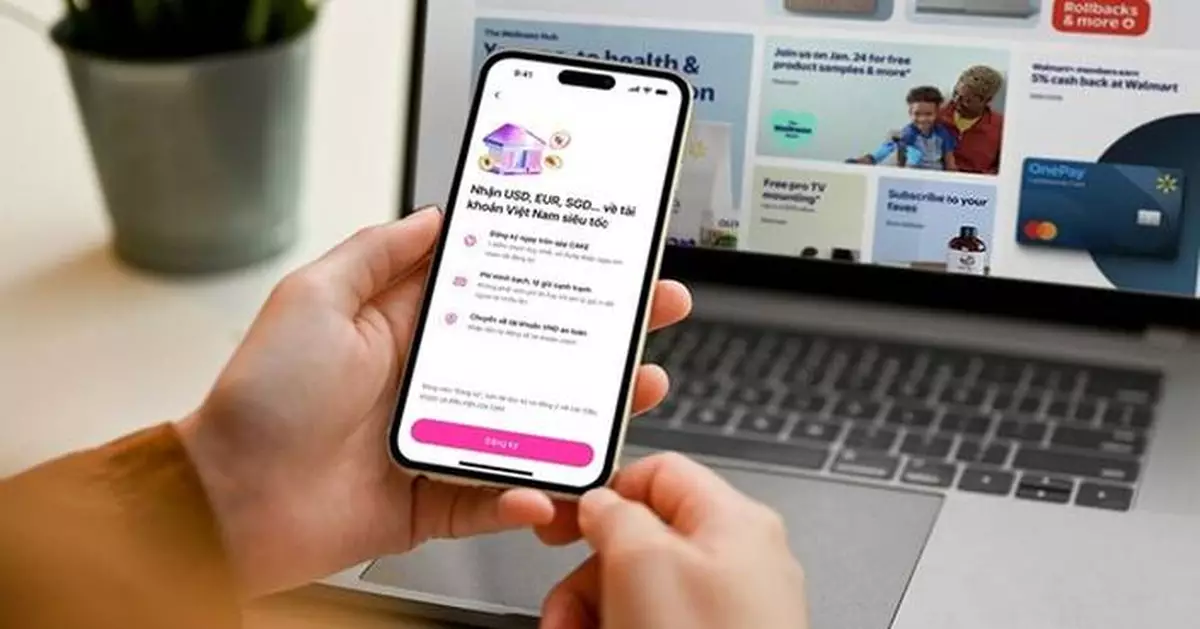
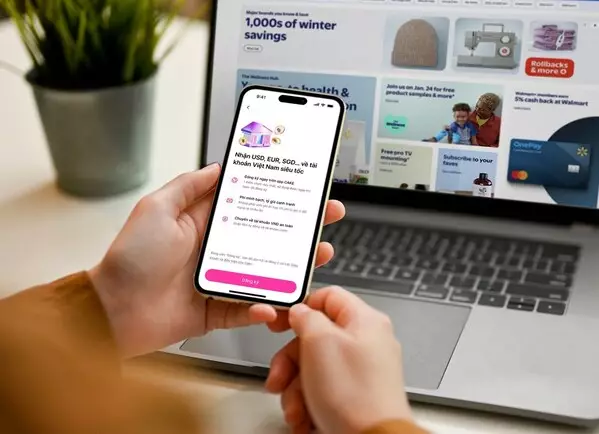
HO CHI MINH CITY, Vietnam, Feb. 6, 2026 /PRNewswire/ -- Cake Digital Bank (Cake) is among the first digital banks in Vietnam to pursue a bank-led approach to cross-border receivables, preparing to roll out a solution in collaboration with Visa as cross-border e-commerce and the digital economy continue to expand across Vietnam and Southeast Asia.
The initiative positions Cake as an early mover among digital banks in Vietnam in bringing cross-border income flows into a fully regulated, onshore digital banking platform—signaling its evolution from a domestically focused digital bank into a financial infrastructure enabler supporting Vietnam's expanding digital economy.
According to Cognitive Market Research, the global cross-border e-commerce market reached USD 791.5 billion in 2024 and is projected to grow at over 30% in the coming years. Vietnam's national e-commerce development plan for 2026–2030 also identifies cross-border e-commerce as a strategic pillar for strengthening the country's digital economy and global competitiveness.
Vietnam is seeing a sharp rise in individuals and small businesses generating income through global platforms such as Amazon, eBay, and Etsy. However, the cross-border receivables landscape remains highly fragmented. Sellers often rely on offshore payment intermediaries, resulting in complex operational processes, extended settlement timelines, and limited transparency around fees and foreign exchange costs.
Against this backdrop, the initiative reflects a broader effort to bring overseas income flows back into Vietnam's regulated banking system. By integrating cross-border receivables within an onshore digital banking framework, it reduces reliance on intermediary layers while improving transparency, settlement efficiency, and oversight of international cash flows.
Mr. Nguyen Huu Quang, CEO of Cake Digital Bank, said: "Cross-border e-commerce is reshaping how individuals and small businesses in Vietnam participate in the global economy. With Cake GlobalX, we are taking an early step toward building bank-native financial infrastructure that supports greater transparency and efficiency in global cash flows."
The collaboration with Visa places the initiative within a global payment framework that meets international standards for security, compliance, and scalability. It reflects a shared commitment to adapting global payment capabilities for emerging digital economies, while maintaining strong regulatory and operational integrity.
Ms. Dang Tuyet Dung, Country Manager, Visa Vietnam and Laos, commented: "Cake's early-mover approach highlights the evolving role of digital banks in expanding cross-border financial access. Visa is pleased to collaborate with innovative institutions to help strengthen global financial connectivity for Vietnam's growing digital economy."
Cake GlobalX is expected to be rolled out to the broader market in the near term, as demand for bank-led, transparent cross-border financial solutions continues to rise across Vietnam and Southeast Asia.
Website: https://cake.vn
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Cake Digital Bank pioneers bank-led cross-border receivables with Visa
|
SEONGNAM, South Korea, Feb. 6, 2026 /PRNewswire/ -- PharmaResearch Co., Ltd. (CEO: Jihoon Sohn) today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for PRD-101, enabling the initiation of a Phase 1 clinical trial in the United States.
PRD-101 is a next-generation nano anticancer drug candidate formulated using nucleotide fragments produced through PharmaResearch's proprietary DOT® (DNA Optimizing Technology). The drug leverages the company's nucleotide-based Advanced DOT™ drug delivery platform, designed to enable efficient loading of therapeutics and improve pharmacokinetics.
The Phase 1 clinical trial will be conducted across up to seven clinical sites in the United States and is expected to enroll approximately 90 patients with locally advanced or metastatic solid tumors. The study is designed to evaluate the safety, tolerability, and pharmacokinetics of PRD-101.
"The FDA clearance of our IND application marks an important milestone for PRD-101. Through this Phase 1 trial, we aim to further characterize PRD-101 and continue advancing our oncology pipeline," PharmaResearch stated.
About PharmaResearch's PRD-101
PRD-101 represents a significant advancement in cancer treatment, utilizing nucleotide fragments produced through PharmaResearch's proprietary DNA optimizing technology (DOT®) in nanoparticle anticancer formulations. Collaborative efforts between PharmaResearch and the University of California Irvine (UCI) researchers, along with support from organizations like the U.S. NCL, have propelled the development of PRD-101. PharmaResearch holds patents and exclusive licenses associated with PRD-101, marking a milestone in the company's innovative endeavors.
Traditional anticancer drugs often face limitations due to high toxicity, which restricts patient eligibility and necessitates careful dosage management. PharmaResearch anticipates that PRD-101 will address these unmet medical needs in anticancer therapy.
About PharmaResearch
PharmaResearch is a pioneering biopharmaceutical company dedicated to enhancing the quality of life through regenerative medicine. With a diverse portfolio that includes medicines, medical devices, cosmetics, and supplements, PharmaResearch focuses on leveraging its core ingredients—DOT® PDRN and DOT ® PN—which are protected by a suite of patents. Headquartered in Gangneung-si, Gangwon-do, South Korea, PharmaResearch also has a subsidiary in Costa Mesa, California.
For more information about PharmaResearch, visit https://pharmaresearch.com/en
SEONGNAM, South Korea, Feb. 6, 2026 /PRNewswire/ -- PharmaResearch Co., Ltd. (CEO: Jihoon Sohn) today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for PRD-101, enabling the initiation of a Phase 1 clinical trial in the United States.
PRD-101 is a next-generation nano anticancer drug candidate formulated using nucleotide fragments produced through PharmaResearch's proprietary DOT® (DNA Optimizing Technology). The drug leverages the company's nucleotide-based Advanced DOT™ drug delivery platform, designed to enable efficient loading of therapeutics and improve pharmacokinetics.
The Phase 1 clinical trial will be conducted across up to seven clinical sites in the United States and is expected to enroll approximately 90 patients with locally advanced or metastatic solid tumors. The study is designed to evaluate the safety, tolerability, and pharmacokinetics of PRD-101.
"The FDA clearance of our IND application marks an important milestone for PRD-101. Through this Phase 1 trial, we aim to further characterize PRD-101 and continue advancing our oncology pipeline," PharmaResearch stated.
About PharmaResearch's PRD-101
PRD-101 represents a significant advancement in cancer treatment, utilizing nucleotide fragments produced through PharmaResearch's proprietary DNA optimizing technology (DOT®) in nanoparticle anticancer formulations. Collaborative efforts between PharmaResearch and the University of California Irvine (UCI) researchers, along with support from organizations like the U.S. NCL, have propelled the development of PRD-101. PharmaResearch holds patents and exclusive licenses associated with PRD-101, marking a milestone in the company's innovative endeavors.
Traditional anticancer drugs often face limitations due to high toxicity, which restricts patient eligibility and necessitates careful dosage management. PharmaResearch anticipates that PRD-101 will address these unmet medical needs in anticancer therapy.
About PharmaResearch
PharmaResearch is a pioneering biopharmaceutical company dedicated to enhancing the quality of life through regenerative medicine. With a diverse portfolio that includes medicines, medical devices, cosmetics, and supplements, PharmaResearch focuses on leveraging its core ingredients—DOT® PDRN and DOT ® PN—which are protected by a suite of patents. Headquartered in Gangneung-si, Gangwon-do, South Korea, PharmaResearch also has a subsidiary in Costa Mesa, California.
For more information about PharmaResearch, visit https://pharmaresearch.com/en
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

PharmaResearch Receives U.S. FDA Clearance to Initiate Phase 1 Clinical Trial of Nano Anticancer Drug, PRD-101