PARIS--(BUSINESS WIRE)--Feb 12, 2025--
Regulatory News:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250211039390/en/
GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported final efficacy and safety results at the conclusion of the REFLECT Phase III clinical trial with LUMEVOQ ® (GS010; lenadogene nolparvovec). The results show that five years after a one-time administration of the gene therapy, the visual acuity improvement among patients with LHON (Leber Hereditary Optic Neuropathy) was sustained while maintaining a favorable safety profile. Bilateral injections provided an additional effect compared to unilateral treatment, demonstrated in some of the responder rate analyses.
“ The latest REFLECT data confirms that the improvement seen with lenadogene nolparvovec is sustained 5 years after treatment has been given, including the additional benefit observed in participants receiving a bilateral intravitreal injection of the gene therapy,” said Prof. Patrick Yu-Wai-Man, MD, PhD, Professor of Ophthalmology and Honorary Consultant Neuro-ophthalmologist at the University of Cambridge, Moorfields Eye Hospital, and the UCL Institute of Ophthalmology, United Kingdom, and International Principal Investigator of REFLECT. “ Importantly, REFLECT participants receiving a bilateral injection had a comparable safety profile to those treated unilaterally. ”
The findings reinforce the results observed at 4 years post-treatment administration, which were reported in March 2024.
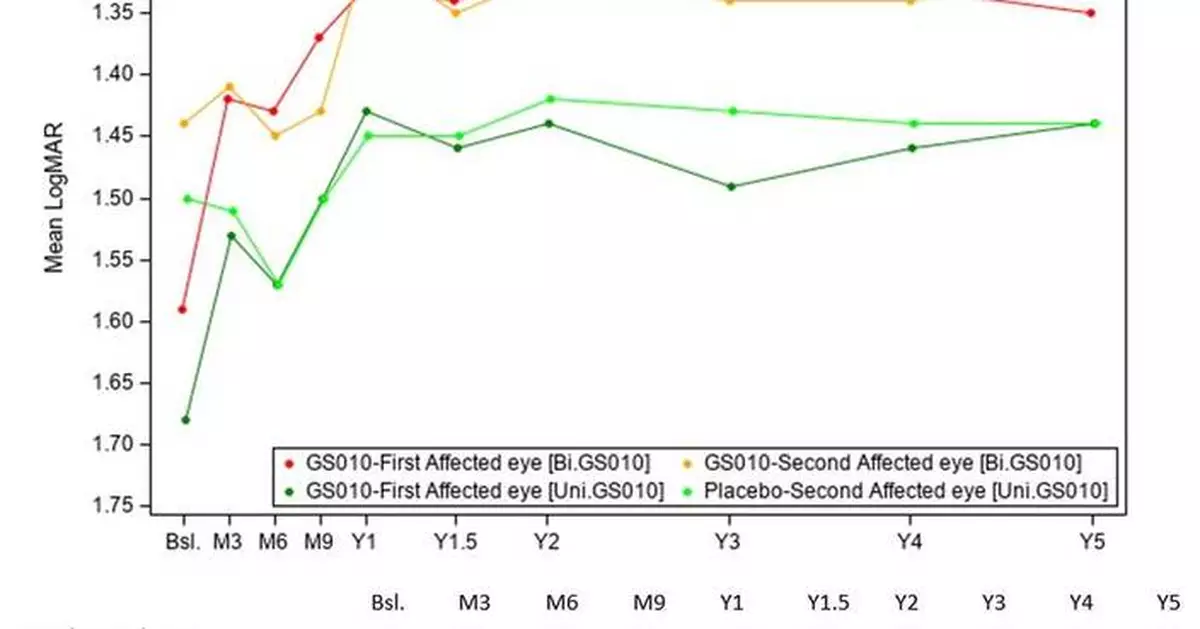
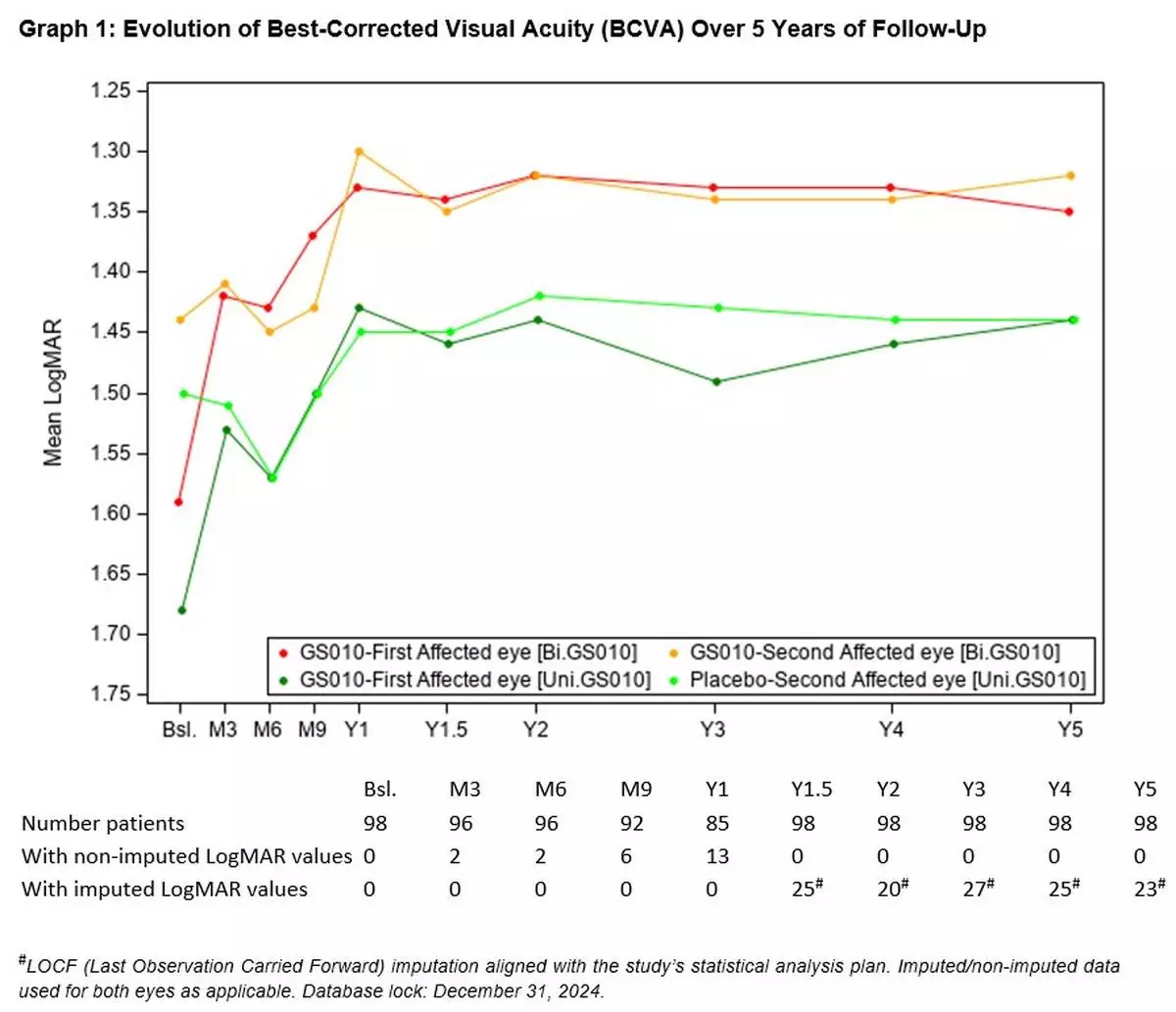
Sustained and meaningful efficacy at Year 5
The evolution of the visual acuity over time shows that visual improvement after lenadogene nolparvovec treatment was maintained over 5 years in all subjects. The improvement of placebo eyes highlights the consistent contralateral treatment effect observed in all clinical trials (which was also documented in sham-treated eyes in the REVERSE 1 and RESCUE 2 trials).
(See Graph 1.)
Because of the severity of the acute phase in LHON, vision could still deteriorate to a low point or nadir in the initial period of the trial. This characteristic of the disease makes the observed nadir (i.e., the worst BCVA recorded from baseline to Year 5) a better reference point to assess the effect of the therapy than baseline vision, which varies greatly depending on the disease stage at the time of enrollment in the study. Relative to the observed nadirs, average visual acuity for all LUMEVOQ-treated eyes increased beyond the +15-letter threshold (-0.3 LogMAR change) that conventionally defines clinically meaningful improvement. (See Table 1.)
Responder analyses reinforce the finding of improved outcomes for patients, for whom natural history typically results in greatly impaired vision with a very low likelihood of spontaneous recovery 3. Five years after injection, patients who were bilaterally treated experienced a higher rate of clinically relevant recovery* from their nadir, compared to patients who had unilateral treatment (75% vs. 60%). 79% of bilaterally treated patients were able to read letters on a screen (on-chart vision), compared to 72% of patients treated in only one eye.
Table 1: Change in Best-Corrected Visual Acuity (BCVA) versus Nadir 5 Years after Injection
Database lock: Dec 31, 2024. Subjects bilaterally treated: 1st affected eyes: n=48; 2 nd affected eyes: n=48; subjects unilaterally treated: 1 st affected eyes: n=50; 2 nd affected eyes: n=50. p<0.0001 for all eye groups using linear mixed model.
Favorable safety profile
The favorable safety profile of LUMEVOQ ® continued to be confirmed, with the safety profile of the drug being demonstrated as comparable in bilaterally and unilaterally treated subjects. There was no study discontinuation related to systemic or ocular adverse events, and there were no serious ocular adverse events. The main ocular adverse event was intraocular inflammation, which was mostly mild and responsive to conventional treatment.
REFLECT was a randomized, double-masked, placebo-controlled Phase III trial involving 98 subjects with vision loss due to LHON caused by a mutated ND4 mitochondrial gene; enrolled ND4 subjects had vision loss up to one year from onset. All subjects received an intravitreal injection (IVT) of lenadogene nolparvovec in their first affected eye. The second affected eye was randomized to either a second IVT of LUMEVOQ ® or a placebo IVT, which was administered on the same day or the following day. 48 subjects were randomized to LUMEVOQ ® bilateral treatment, and 50 to lenadogene nolparvovec unilateral treatment (first-affected eye treated with LUMEVOQ ®, second-affected eye treated with placebo). REFLECT patients were followed up to 5 years post-injection.
* “Clinically Relevant Recovery”, or CRR, refers to an improvement in Best-Corrected Visual Acuity (BCVA) that satisfies one of two conditions: (1) A 10-letter (≥0.2 LogMAR) improvement for an on-chart starting visual acuity. (2) Improvement from “off-chart” to “on-chart” (≤1.6 LogMAR).
References:
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1 st eye, with the 2 nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of subjects have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously.
About LUMEVOQ ® (GS010; lenadogene nolparvovec)
LUMEVOQ ® (GS010; lenadogene nolparvovec) targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function. “LUMEVOQ” was accepted as the invented name for GS010 (lenadogene nolparvovec) by the European Medicines Agency (EMA) in October 2018. LUMEVOQ® (GS010; lenadogene nolparvovec) has not been registered in any country at this stage.
About REFLECT
REFLECT was a multi-center, randomized, double-masked, placebo-controlled study to evaluate the safety and efficacy of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 ( ND4 ) mutation. In the active arm, GS010 was administered as a single intravitreal injection in each eye of each subject. In the placebo arm, GS010 was administered as a single intravitreal injection to the first affected eye, while the fellow eye received a placebo injection.
The primary endpoint for the REFLECT trial was the BCVA reported in LogMAR at 1.5 years (78 weeks) post-treatment in the second‑affected/not‑yet‑affected eye. The change from baseline in second‑affected/not‑yet‑affected eyes receiving GS010 and placebo was the primary response of interest. The secondary efficacy endpoints included: change from baseline in BCVA reported in LogMAR at 2, 3, 4 and 5 years post-treatment in the second‑affected/not‑yet‑affected eye compared to both placebo and the first‑affected eye receiving GS010, change from baseline in OCT and contrast sensitivity as well as quality of life scales.
The trial was conducted in multiple centers across Europe/UK (1 each in France, Spain, Italy and the UK), the US (6 centers) and Taiwan (1 center). The trial planned to enroll 90 subjects with vision loss up to 1 year in duration; 98 subjects were successfully screened and treated. The first subject was treated in March 2018 and the last one in July 2019. Long-term follow-up of the last patient was completed on July 23, 2024.
ClinicalTrials.gov Identifiers:
REFLECT: NCT03293524

Evolution of Visual Acuity in the REFLECT Phase III Study (Graphic: Business Wire)