|
- Okto Platform Live On Testnet: Pioneering blockchain adoption and enabling seamless cross-chain transactions across major networks like HyperLiquid, Aptos, Solana, and EVM.
- The live testnet link for Okto: https://testnet.okto.tech/
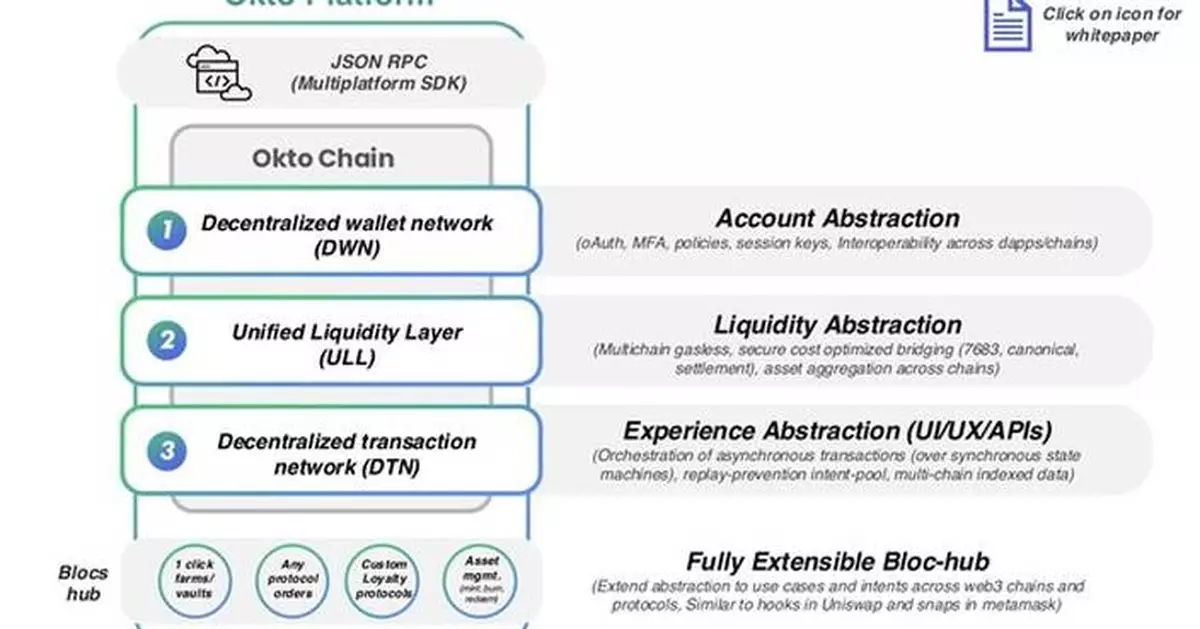
BENGALURU, India, Feb. 22, 2025 /PRNewswire/ -- Okto, a complete chain abstraction platform, is thrilled to announce the official launch of Okto Platform Testnet - the industry's first Complete end-to-end chain abstraction solution. This groundbreaking solution is set to redefine the landscape of blockchain interoperability by simplifying decentralized application (dApp) development, cross-chain transactions, and multi-chain innovation. The platform enables seamless communication across a range of leading blockchain ecosystems, such as all EVM chains, HyperLiquid (HL), Aptos, Solana, and Cosmos chains/ecosystems.
Okto's chain abstraction technology is already delivering impressive results, with real-world success stories across gaming, social and DeFi applications that showcase its efficiency and transformative potential. The Okto Wallet, has facilitated the creation of over 12 million wallets, integrated more than 50 protocols, and supports over 20 EVM and Alt-VM chains. Okto has already clocked in $1 billion in Monthly Recurring Revenue.
Neeraj Khandelwal, Co-Founder of Okto & CoinDCX, said "We have been developing our Chain Abstraction technology for over two years, collaborating closely with prominent blockchain networks to rigorously test cross-chain transactions, liquidity, and interoperability. The results have been exceptional. Okto's core mission has always been to simplify the user experience, ensuring it mirrors the simplicity and efficiency of Web2 with just a single click. We are now empowering developers with the ability to seamlessly integrate Web3 functionalities into their applications with unprecedented ease and efficiency."
Rohit Jain, Head of DeFi Initiatives at Okto and CoinDCX, said: "We built a world-class team and collaborated with industry leaders such as Nethermind, Silence Laboratories, Across Protocol, and Agoric. By leveraging cutting-edge security from EigenLayer and Polygon CDK, each partnership brought vital expertise to our mission. Today, we stand at a pivotal moment with the launch of the Okto Platform Testnet."
"While collaborating with multiple networks co-building on the Okto platform, one key piece of feedback from developers has been the significant reduction in development time—by over 90%. Okto does the heavy lifting of abstracting Web3 complexities across fragmented ecosystems and empowers developers to offer their end users a frictionless, single-click experience. This allows developers to focus on their core product."
- Okto Platform Live On Testnet: Pioneering blockchain adoption and enabling seamless cross-chain transactions across major networks like HyperLiquid, Aptos, Solana, and EVM.
- The live testnet link for Okto: https://testnet.okto.tech/
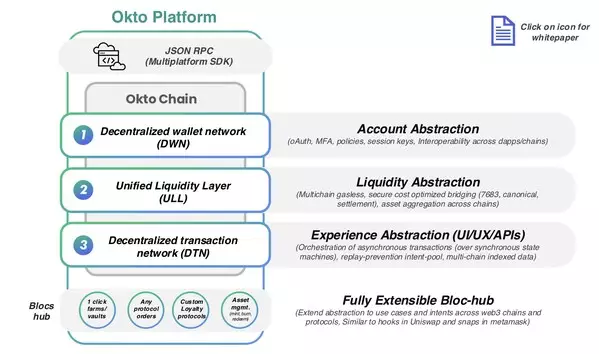
BENGALURU, India, Feb. 22, 2025 /PRNewswire/ -- Okto, a complete chain abstraction platform, is thrilled to announce the official launch of Okto Platform Testnet - the industry's first Complete end-to-end chain abstraction solution. This groundbreaking solution is set to redefine the landscape of blockchain interoperability by simplifying decentralized application (dApp) development, cross-chain transactions, and multi-chain innovation. The platform enables seamless communication across a range of leading blockchain ecosystems, such as all EVM chains, HyperLiquid (HL), Aptos, Solana, and Cosmos chains/ecosystems.
Okto's chain abstraction technology is already delivering impressive results, with real-world success stories across gaming, social and DeFi applications that showcase its efficiency and transformative potential. The Okto Wallet, has facilitated the creation of over 12 million wallets, integrated more than 50 protocols, and supports over 20 EVM and Alt-VM chains. Okto has already clocked in $1 billion in Monthly Recurring Revenue.
Neeraj Khandelwal, Co-Founder of Okto & CoinDCX, said "We have been developing our Chain Abstraction technology for over two years, collaborating closely with prominent blockchain networks to rigorously test cross-chain transactions, liquidity, and interoperability. The results have been exceptional. Okto's core mission has always been to simplify the user experience, ensuring it mirrors the simplicity and efficiency of Web2 with just a single click. We are now empowering developers with the ability to seamlessly integrate Web3 functionalities into their applications with unprecedented ease and efficiency."
Rohit Jain, Head of DeFi Initiatives at Okto and CoinDCX, said: "We built a world-class team and collaborated with industry leaders such as Nethermind, Silence Laboratories, Across Protocol, and Agoric. By leveraging cutting-edge security from EigenLayer and Polygon CDK, each partnership brought vital expertise to our mission. Today, we stand at a pivotal moment with the launch of the Okto Platform Testnet."
"While collaborating with multiple networks co-building on the Okto platform, one key piece of feedback from developers has been the significant reduction in development time—by over 90%. Okto does the heavy lifting of abstracting Web3 complexities across fragmented ecosystems and empowers developers to offer their end users a frictionless, single-click experience. This allows developers to focus on their core product."
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Okto, the first end-to-end chain abstraction solution for developers, promises 90% reduction in development time, now live on Testnet
RIYADH, Saudi Arabia, Jan. 28, 2026 /PRNewswire/ -- The Ministry of Industry and Mineral Resources has announced the successful conclusion of the fifth edition of the Future Minerals Forum (FMF), with a record attendance of 21,500 participants, including investment leaders, heads of major mining companies, experts, and technical specialists from around the world.
The Forum, held at the King Abdulaziz International Conference Center in Riyadh from January 13 to 15, 2026, witnessed the signing of 132 agreements and Memoranda of Understanding totaling USD 26.6 billion. These covered critical areas including exploration and mining, financing, research and development, innovation, sustainability, value-added supply chains, and mining industries.
In his closing remarks, His Excellency the Minister of Industry and Mineral Resources, Bandar Alkhorayef, noted that the unprecedented momentum witnessed reflects progress across the broader global mining ecosystem. He emphasized that within five years, the Forum has advanced at a remarkable pace, becoming a shared cause for all stakeholders and laying the foundation for year-round collaboration.
Alkhorayef highlighted that real progress is linked to accelerating the adoption of mining technologies as a key driver of growth. He noted the Kingdom's efforts to reform its regulatory framework and improve the investment environment by reviewing procedures, updating regulations, streamlining licensing processes, providing accessible geological data, and launching incentive programs to support exploration and attract partners across the entire value chain.
Over three days, more than 450 speakers - including ministers, government representatives, and industry experts - explored investment partnerships, community development, new mining projects, AI-enabled decision-making, and integrating local economies into the mining value chain.
The international exhibition featured 274 exhibitors and 13 official country pavilions from Australia, Canada, the United Kingdom, Germany, France, Sweden, Austria, Brazil, Egypt, Morocco, Pakistan, Sudan, and Mauritania. The exhibition was organized across four zones: heavy machinery and equipment, cutting-edge technology innovations, major global companies, and junior exploration companies.
About the Future Minerals Forum
The Future Minerals Forum (FMF), launched in 2022, is the world's premier platform for the minerals industry. FMF brings together governments, international organizations, and stakeholders to shape the future of the minerals industry. Through the Ministerial Roundtable, conference, and exhibition, the global conversation on minerals takes center stage, driving innovation and collaboration toward a sustainable future.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

5th Future Minerals Forum Concludes with 21,500 Attendees, Agreements Worth USD 26.6 Billion