LOS ANGELES--(BUSINESS WIRE)--May 5, 2025--
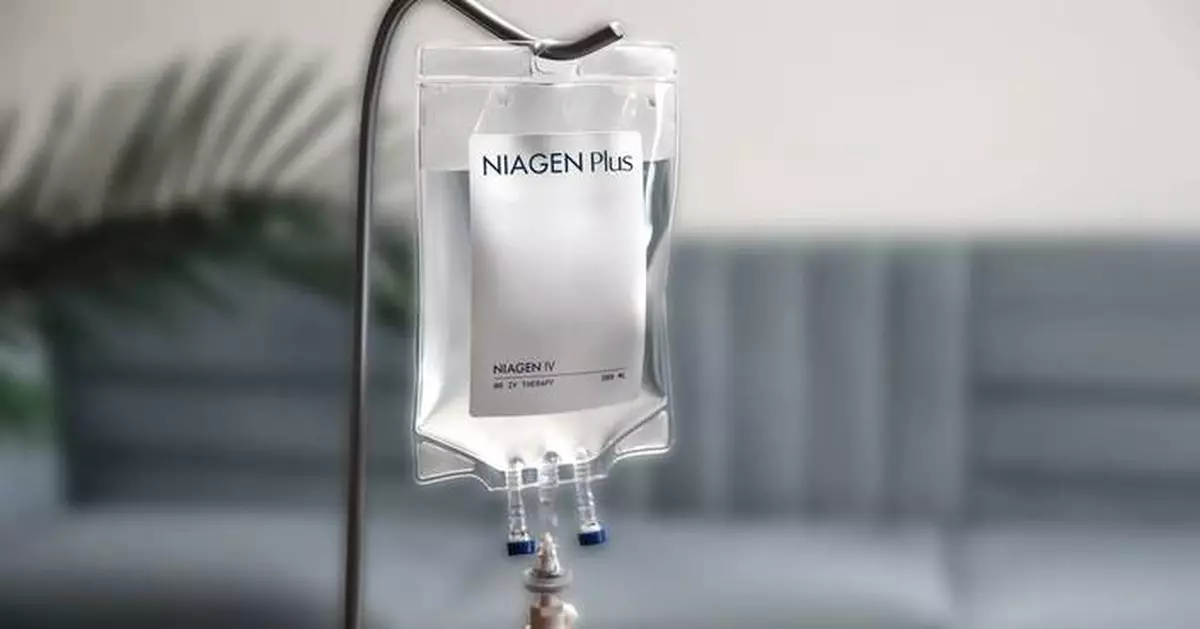
Niagen Bioscience, Inc. (Nasdaq: NAGE) (formerly ChromaDex Corp.), the global authority on NAD+ (nicotinamide adenine dinucleotide) with a focus on the science of healthy aging, today announced the continued expansion of its clinical footprint with additional new clinics now offering pharmaceutical-grade Niagen ® IV and injections featuring patented nicotinamide riboside. These new providers join a growing network of almost 600 clinics nationwide offering the first-of-its-kind, pharmaceutical-grade NAD-boosting Niagen IV therapy. For a list of all clinic providers, please visit https://www.niagenplus.com/pages/clinic-locator.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250505444041/en/
Rob Fried, CEO of Niagen Bioscience, stated, “At the heart of the Niagen Plus ecosystem are our sterile pharmaceutical-grade Niagen NR formulations for IV as well as intramuscular (IM) and subcutaneous (SubQ) injections. These Niagen NR products are high-quality solutions for those seeking the most advanced, clinically supported NAD-boosting interventions.”
Some of the new clinic providers include:
Outperforming NAD+ IV, Niagen IV is a significant advancement in NAD-boosting therapy. In a head-to-head comparison with NAD+ IV, Niagen IV offers superior tolerability, a 75% shorter infusion time, and a statistically significant 20% increase in whole blood NAD+ levels three hours after infusion, as measured by NAD+ dried blood spot tests ( MedRxiv ). Study results demonstrated that NAD+ IV had a longer infusion time and was associated with a high prevalence of uncomfortable side effects such as headaches, stomach pain, diarrhea, and nausea, which were not observed with Niagen IV.
A common misconception is that the NAD+ molecule itself is bioavailable and that consumers can experience elevated NAD+ levels by taking NAD+ itself orally or intravenously. As a large, phosphorylated molecule, the NAD+ molecule cannot cross the cell membrane directly and must first be broken down into other NAD+ precursors, such as Niagen NR. Among various NAD+ precursors and the NAD+ molecule itself, Niagen is most efficient and effective at increasing NAD+ levels—it easily crosses the cell membrane directly and requires fewer steps for conversion into NAD+.
Pharmaceutical-grade Niagen is compounded and distributed by 503B registered outsourcing facilities, including Wells Pharma of Houston, which adhere to stringent testing standards as required by the U.S. FDA, which ensures compliance with US Pharmacopoeia (USP) particulate matter and bacterial endotoxin guidelines. For more information on regulatory compliance and the scientific and quality standards behind Niagen, visit niagenbioscience.com.
Arete Wellness, Beyond Remedi, Clarus Health, Energy4Life Centers, LifeMed Institute, Navo, and PEAQ Society highlight the benefits of Niagen IV:
For more information about Niagen IV, visit www.niagenplus.com.
About Niagen Bioscience:
Niagen Bioscience, Inc. (NASDAQ: NAGE), formerly ChromaDex Corp., is the global leader in NAD+ (nicotinamide adenine dinucleotide) science and healthy-aging research. As a trusted pioneer of NAD+ discoveries, Niagen Bioscience is dedicated to advancing healthspan through precision science and innovative NAD+-boosting solutions.
The Niagen Bioscience team, composed of world-renowned scientists, works with independent investigators from esteemed universities and research institutions around the globe to uncover the full potential of NAD+. A vital coenzyme found in every cell of the human body, NAD+ declines with age and exposure to everyday lifestyle stressors. NAD+ depletion is a key contributor to age-related changes in health and vitality.
Distinguished by state-of-the-art laboratories, rigorous scientific and quality protocols, and collaborations with leading research institutions worldwide, Niagen Bioscience sets the gold standard for research, quality, and innovation. There’s a better way to age.
At the heart of its clinically proven product portfolio is Niagen ® (patented nicotinamide riboside, or NR), the most efficient, well-researched, high-quality, and legal NAD+ booster available. Niagen powers the Company’s consumer supplement, Tru Niagen ®, the number one NAD+ boosting oral supplement in the United States † (available at www.truniagen.com ), and Niagen Plus™, featuring pharmaceutical-grade intravenous (IV) and injectable Niagen products ( www.niagenplus.com ). Pharmaceutical-grade Niagen IV and injections are compounded and distributed by U.S. FDA-registered 503B outsourcing facilities and are available exclusively at clinics with a prescription.
Niagen Bioscience’s robust patent portfolio protects NR and other NAD+ precursors. Niagen Bioscience maintains a website at www.niagenbioscience.com, where copies of press releases, news, and financial information are regularly published.
Forward-Looking Statements:
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Statements that are not a description of historical facts constitute forward-looking statements and may often, but not always, be identified by the use of such words as “expects,” “anticipates,” “intends” “estimates,” “plans,” “potential,” “possible,” “probable,” “believes” “seeks,” “may,” “will,” “should,” “could,” “predicts,” “projects,” “continue,” “would” or the negative of such terms or other similar expressions.
Risks that contribute to the uncertain nature of the forward-looking statements include: inflationary conditions and adverse economic conditions; our history of operating losses and need to obtain additional financing; the growth and profitability of our product sales; our ability to maintain and grow sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third-party suppliers; risks of unanticipated developments in and risks related to the Company’s ability to secure adequate quantities of pharmaceutical-grade Niagen in a timely manner; the Company’s ability to obtain appropriate contracts and arrangements with U.S. FDA-registered 503B outsourcing facilities required to compound and distribute pharmaceutical-grade Niagen to clinics; the Company’s ability to remain on the U.S. FDA Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Category 1 list; the Company’s ability to maintain and enforce the Company’s existing intellectual property and obtain new patents; whether the potential benefits of NRC can be further supported; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities; and the risks and uncertainties associated with our business and financial condition in general. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company’s most recent Annual Report on Form 10-K, the Company’s Quarterly Reports on Form 10-Q and other filings submitted by the Company to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and actual results may differ materially from those suggested by these forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement and Niagen Bioscience undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof.
†Based on the top-selling dietary supplement brands by revenue per the largest U.S. e-commerce marketplace (as of 1/1/2024 - 12/31/2024).
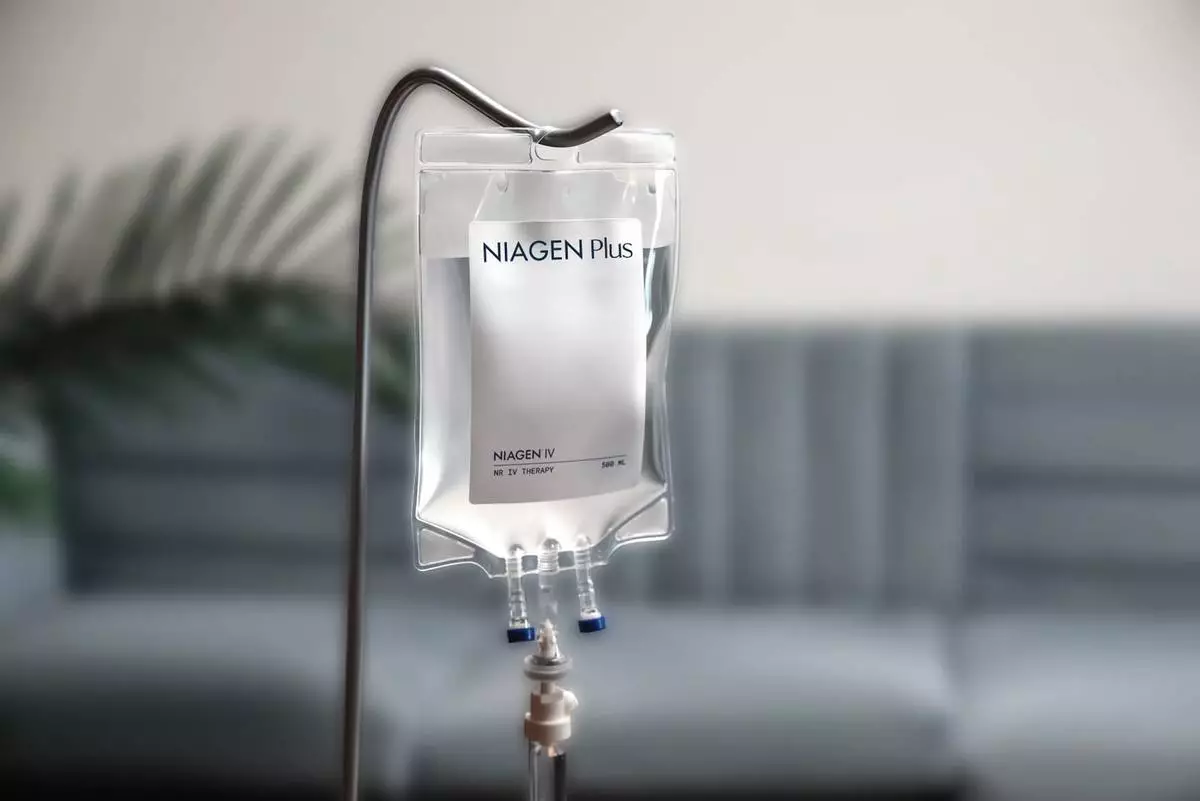

Pharmaceutical-Grade Niagen® IV Is Now Available in Almost 600 Clinics Nationwide