BARCELONA, Spain--(BUSINESS WIRE)--May 6, 2025--
INBRAIN Neuroelectronics, a clinical-stage neurotechnology company developing precision brain-computer interfaces (BCIs) powered by graphene, announced today it has been awarded a €4 million grant by the Spanish Ministry of Industry and Tourism through the PERTE Chip initiative. The grant will accelerate INBRAIN’s development of brain-computer interface technology that integrates intelligent computing and graphene-based materials to decode and modulate real time brain activity for therapeutic purposes.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250505249158/en/
The PERTE Chip initiative - “Strategic Project for Economic Recovery and Transformation of Microelectronics and Semiconductors” - aims to strengthen the design and production capabilities of the microelectronics and semiconductor industry in Spain from a comprehensive perspective, while promoting strategic technological autonomy for both Spain and the European Union.
The Ministry provisionally awarded €53.2 million in funding on May 5, 2025, to support 37 breakthrough projects across 11 autonomous communities. INBRAIN’s project was one of several selected for their high-impact potential in areas including disruptive materials, semiconductor manufacturing, cybersecurity, and AI-based computing tools.
“We are honored to receive this national support as part of Spain’s effort to lead in cutting-edge neurotechnology,” said Carolina Aguilar, CEO and Co-Founder of INBRAIN Neuroelectronics. “This grant will enable us to accelerate our mission to develop precision neurotechnologies that can transform the treatment of neurological diseases, while also helping to position Spain at the forefront of deep tech innovation in healthcare.”
“INBRAIN Neuroelectronics represents exactly the kind of cutting-edge innovation we aim to support from the Government of Catalonia,” said Mr Miquel Sàmper, regional Minister of Business and Labor. “By combining advanced materials like graphene and neurotechnology, INBRAIN is not only pushing the boundaries of healthcare innovation, but also strengthening Catalan, Spain and Europe’s leadership in strategic deep tech sectors. Supporting projects like this is essential to building the country’s technological autonomy and long-term competitiveness in the medtech industry.”
INBRAIN’s technology platform uses graphene neural interfaces to deliver ultra-precise, adaptive, and biocompatible neuromodulation for a range of conditions including, Parkinson’s disease, stroke rehabilitation, epilepsy and in the future neuropsychiatric disorders.
About INBRAIN Neuroelectronics
INBRAIN Neuroelectronics is pioneering real-time precision neurology with the world’s first graphene-based brain-computer interface therapeutics (BCI-Tx) platform. Our technology combines advanced neural decoding and micrometric modulation to deliver personalized, adaptive treatments for conditions like Parkinson’s, epilepsy, and stroke rehabilitation. By continuously monitoring and adjusting therapies in real-time, our AI-driven platform enhances outcomes while reducing side effects. This pioneering and disruptive development has led to a FDA Breakthrough Device Designation for INBRAIN’s BCI-Tx in Parkinson’s Disease. In collaboration with partners like Merck KGaA and our subsidiary INNERVIA Bioelectronics, we are expanding these innovations to treat peripheral nerve and systemic diseases, driving the future of neurotechnology and bioelectronics. Visit us at www.inbrain-neuroelectronics.com.

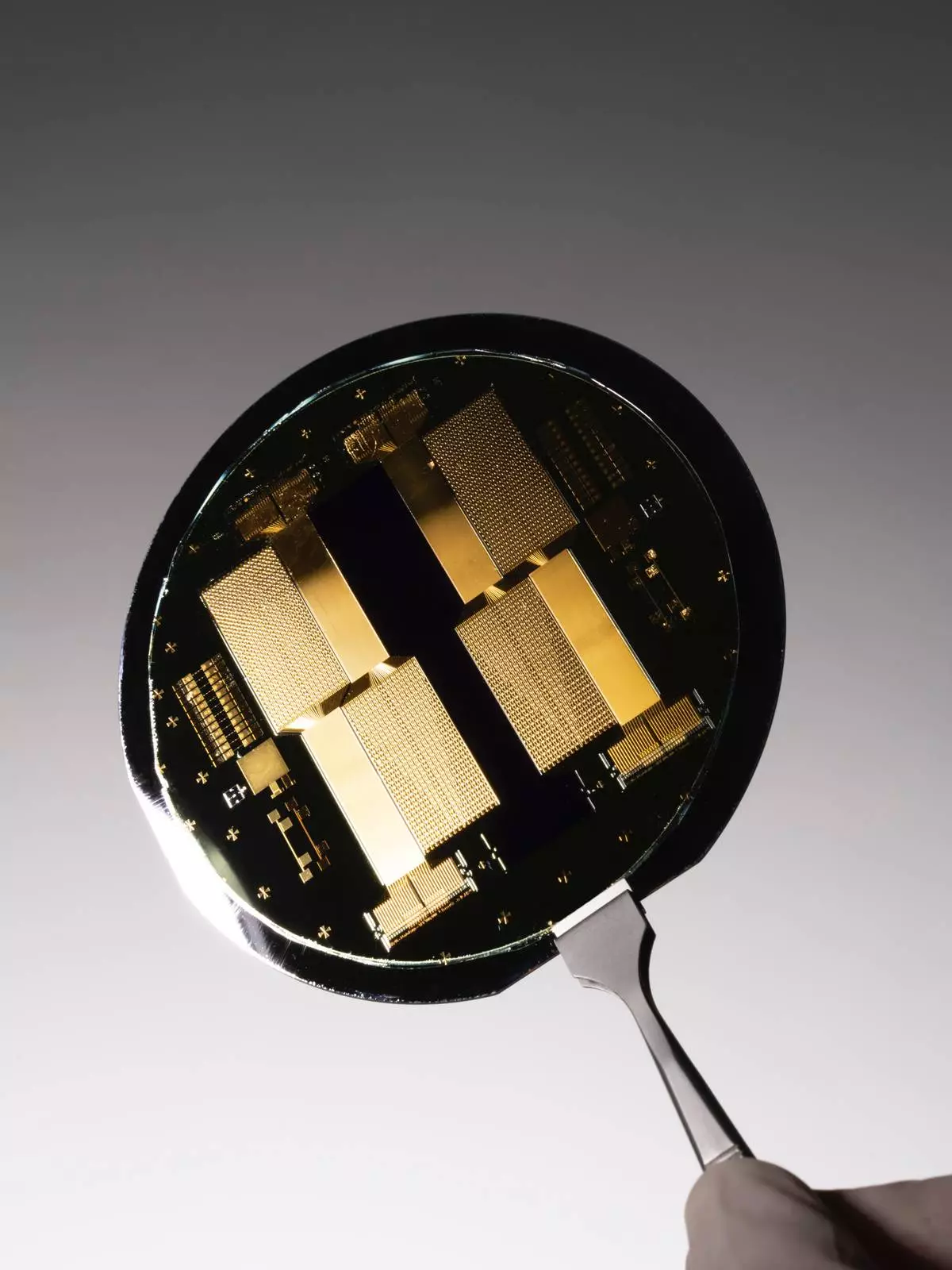
INBRAIN Neuroelectronics graphene-based brain-computer interface semiconductor technology (Photo credit: INBRAIN Neuroelectronics).

Photo credit: INBRAIN Neuroelectronics
LONDON (AP) — King Charles III has been “deeply touched” by the response to his update on his cancer treatment, Buckingham Palace said Saturday, adding that the monarch is pleased to have highlighted the value of screening programs for the disease.
Medics and health charities have praised the king for his openness, saying his statement on Friday had already prompted people to seek information about cancer.
In a strikingly personal video statement, the British monarch acknowledged that a cancer diagnosis can feel “overwhelming,” but said catching the disease early brings “the precious gift of hope.”
Here’s what to know about the king's condition and his message.
The 77-year-old king said in a statement broadcast Friday that his treatment schedule will be reduced in the new year, “thanks to early diagnosis, effective intervention and adherence to ‘doctors’ orders.’”
He encouraged others to take advantage of screening programs such as those for breast, bowel and cervical cancer offered by Britain’s public health service.
“Early diagnosis quite simply saves lives,” the king said in the statement aired during a “Stand Up to Cancer” telethon on TV station Channel 4. He said catching the disease early had allowed him “to continue leading a full and active life even while undergoing treatment.”
Charles has received outpatient treatment for almost two years. Buckingham Palace did not say the king is in remission, but that his treatment is moving to a “precautionary phase” and his condition will be monitored to ensure his continued recovery.
“I know from my own experience that a cancer diagnosis can feel overwhelming,” the king said in his video statement. “Yet I also know that early detection is the key that can transform treatment journeys, giving invaluable time to medical teams – and, to their patients, the precious gift of hope.”
Charles announced in February 2024 that he had been diagnosed with cancer, and, in a break from centuries of secrecy about royal health, he has since spoken about the illness, using his story to promote cancer awareness and treatment.
The openness has limits, though. The king has not disclosed what type of cancer he has or what kind of treatment he is receiving. The palace said it was an intentional decision designed to ensure his message reaches the widest possible audience.
The king’s cancer was discovered after treatment for an enlarged prostate. While doctors ruled out prostate cancer, tests revealed “a separate issue of concern,” palace officials said last year.
Charles suspended his public appearances for about two months after his diagnosis. Since returning to the public eye, he has visited cancer treatment centers across the country and shared stories with fellow patients.
Buckingham Palace said Charles “will be greatly encouraged and deeply touched by the very positive reaction" his message has generated. “He will be particularly pleased at the way it has helped to shine a light on the benefits of cancer screening programs,” it added.
British cancer charities said the number of people seeking information about cancer jumped after the king revealed he was undergoing treatment last year.
Cancer Research U.K. said about 100,000 people have visited its Screening Checker website since it was launched on Dec. 5, most of them since the king’s statement on Friday.
The charity's Chief Executive Michelle Mitchell said: “When public figures speak openly about their cancer diagnosis, it can prompt others to check in on their health.”
Broadcaster Jonathan Dimbleby, the king’s authorized biographer, said the statement was “a remarkable thing for a monarch to do.”
“It takes guts, and the fact that he came out and did that will save lives,’” Dimbleby said.
The Princess of Wales, who announced her own cancer diagnosis six weeks after her father-in-law, has also given updates on her treatment. Kate announced in January that her cancer is in remission.
Find more of AP’s coverage at https://apnews.com/hub/royalty

Britain's King Charles III attends an Advent Service at Westminster Abbey, in London, Wednesday, Dec. 10, 2025. (Chris Jackson/Pool Photo via AP)

Britain's King Charles III attends an Advent Service at Westminster Abbey, in London, Wednesday, Dec. 10, 2025. (Chris Jackson/Pool Photo via AP)














