|
BOSTON, Sept. 25, 2025 /PRNewswire/ -- Bambusa Therapeutics, Inc. (Bambusa Therapeutics), a clinical-stage biotechnology company pioneering next-generation bispecific antibodies for immunology and inflammation, today announced two landmark achievements for its lead program, BBT001.
Last week, Bambusa Therapeutics unveiled highly positive results from the single ascending dose portion of its ongoing Phase I healthy volunteer study of BBT001 at the European Academy of Dermatology and Venereology (EADV) 2025.
The data demonstrated:
- A favorable safety and tolerability profile across all dose levels with no dose-limiting adverse events.
- A best-in-class pharmacokinetic profile (~33-day half-life), validating Bambusa's half-life extension technology and supporting flexible dosing regimens.
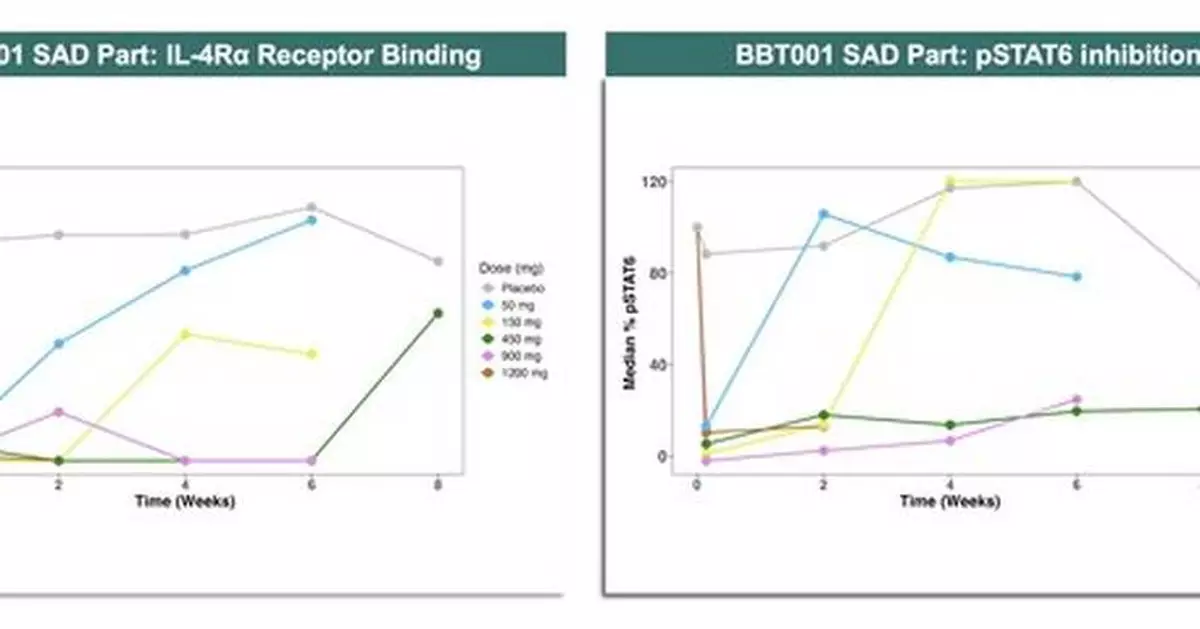
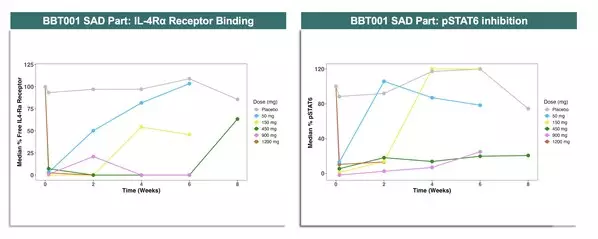
- Rapid, complete, and sustained IL-4Rα binding and pSTAT6 inhibition observed through Week 8+ following a single dose of BBT001.
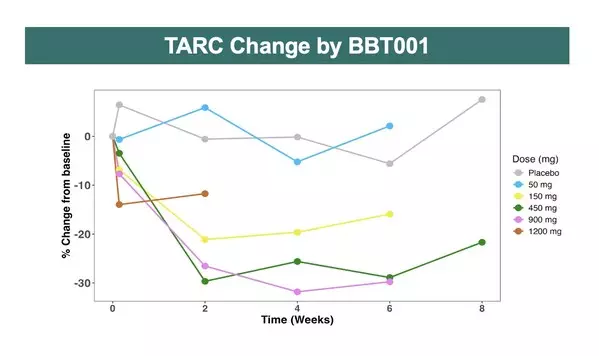
- An unprecedented, dose-dependent, rapid, deep, and sustained reduction in TARC levels after a single dose of BBT001, with potent suppression maintained through Week 8+.
"These results provide the first clinical evidence of BBT001's potential to become a best-in-disease biologic in atopic dermatitis," said Thang Ho, Ph.D., Senior Vice President of Development Sciences at Bambusa Therapeutics. "Despite low baseline TARC levels, we observed an unprecedented dose-dependent reduction in healthy volunteers, suggesting synergy between IL-4Rα and IL-31 inhibition. Given the strong correlation between TARC reduction and treatment efficacy in atopic dermatitis, these data give us confidence that BBT001 may deliver faster onset and deeper relief for patients across the Type 2 inflammatory skin disease spectrum."
Today, Bambusa Therapeutics has achieved its next major milestone: the dosing of the first patient with moderate-to-severe atopic dermatitis in the Phase I clinical trial of BBT001.
"Achieving patient dosing within 16 months of company inception underscores our pace of execution and represents a powerful moment for Bambusa Therapeutics and the atopic dermatitis community.", said Shanshan Xu, M.D., Ph.D., Founder & Chief Executive Officer of Bambusa Therapeutics. "With the trial now underway, we feel a deep responsibility to patients to accelerate development, generate proof-of-concept clinical data in patients, challenge and ultimately redefine the standard of care in atopic dermatitis."
About the BBT001-001 Clinical Trial
BBT001-001 is a randomized, placebo-controlled, single- and multiple-ascending-dose study in healthy volunteers and adults with moderate to severe atopic dermatitis. The trial is evaluating safety, tolerability, pharmacokinetics, immunogenicity, pharmacodynamics, and preliminary clinical activity. Patient enrollment is ongoing, with additional data expected in 2026.
About BBT001
BBT001 is Bambusa Therapeutics' lead program and a first-in-class, multi-targeting, half-life extended bispecific antibody engineered to block both IL-4Rα and IL-31 signaling. By addressing type 2 inflammation and directly targeting the pathways that drive itch, BBT001 is designed to provide faster and more durable relief for patients with atopic dermatitis and other inflammatory skin diseases. The molecule has demonstrated a favorable safety profile and strong pharmacokinetics in healthy volunteers, supporting its advancement into patient studies.
About Bambusa Therapeutics, Inc.
Bambusa Therapeutics is a clinical-stage biotechnology company building a portfolio designed to transform care across a wide spectrum of chronic disease. Powered by an innovative antibody engineering platform enhanced with half-life extension and high-concentration subcutaneous delivery, the company's vision is to deliver transformative medicines for patients across every stage of life, setting the pace for the next era of I&I therapeutics. Headquartered in Boston, Bambusa Therapeutics' pipeline is designed to achieve best-in-disease impact across multiple therapeutic areas:
- BBT001 is a first-in-class half-life extended bispecific antibody targeting IL-4Rα and IL-31, currently in Phase 1 clinical development for atopic dermatitis and other Type 2 inflammatory skin diseases.
- BBT002 is a first-in-class half-life extended bispecific antibody targeting IL-4Rα and IL-5, in Phase 1 clinical development for Type 2 inflammatory disorders including COPD, asthma, and chronic rhinosinusitis with nasal polyps (CRSwNP).
- BBT003 and BBT004 are preclinical programs focused on gastroenterology, including inflammatory bowel disease (IBD), and rheumatological diseases, respectively.
For more information, visit www.bambusatx.com.
BOSTON, Sept. 25, 2025 /PRNewswire/ -- Bambusa Therapeutics, Inc. (Bambusa Therapeutics), a clinical-stage biotechnology company pioneering next-generation bispecific antibodies for immunology and inflammation, today announced two landmark achievements for its lead program, BBT001.
Last week, Bambusa Therapeutics unveiled highly positive results from the single ascending dose portion of its ongoing Phase I healthy volunteer study of BBT001 at the European Academy of Dermatology and Venereology (EADV) 2025.
The data demonstrated:
- A favorable safety and tolerability profile across all dose levels with no dose-limiting adverse events.
- A best-in-class pharmacokinetic profile (~33-day half-life), validating Bambusa's half-life extension technology and supporting flexible dosing regimens.
- Rapid, complete, and sustained IL-4Rα binding and pSTAT6 inhibition observed through Week 8+ following a single dose of BBT001.
- An unprecedented, dose-dependent, rapid, deep, and sustained reduction in TARC levels after a single dose of BBT001, with potent suppression maintained through Week 8+.
"These results provide the first clinical evidence of BBT001's potential to become a best-in-disease biologic in atopic dermatitis," said Thang Ho, Ph.D., Senior Vice President of Development Sciences at Bambusa Therapeutics. "Despite low baseline TARC levels, we observed an unprecedented dose-dependent reduction in healthy volunteers, suggesting synergy between IL-4Rα and IL-31 inhibition. Given the strong correlation between TARC reduction and treatment efficacy in atopic dermatitis, these data give us confidence that BBT001 may deliver faster onset and deeper relief for patients across the Type 2 inflammatory skin disease spectrum."
Today, Bambusa Therapeutics has achieved its next major milestone: the dosing of the first patient with moderate-to-severe atopic dermatitis in the Phase I clinical trial of BBT001.
"Achieving patient dosing within 16 months of company inception underscores our pace of execution and represents a powerful moment for Bambusa Therapeutics and the atopic dermatitis community.", said Shanshan Xu, M.D., Ph.D., Founder & Chief Executive Officer of Bambusa Therapeutics. "With the trial now underway, we feel a deep responsibility to patients to accelerate development, generate proof-of-concept clinical data in patients, challenge and ultimately redefine the standard of care in atopic dermatitis."
About the BBT001-001 Clinical Trial
BBT001-001 is a randomized, placebo-controlled, single- and multiple-ascending-dose study in healthy volunteers and adults with moderate to severe atopic dermatitis. The trial is evaluating safety, tolerability, pharmacokinetics, immunogenicity, pharmacodynamics, and preliminary clinical activity. Patient enrollment is ongoing, with additional data expected in 2026.
About BBT001
BBT001 is Bambusa Therapeutics' lead program and a first-in-class, multi-targeting, half-life extended bispecific antibody engineered to block both IL-4Rα and IL-31 signaling. By addressing type 2 inflammation and directly targeting the pathways that drive itch, BBT001 is designed to provide faster and more durable relief for patients with atopic dermatitis and other inflammatory skin diseases. The molecule has demonstrated a favorable safety profile and strong pharmacokinetics in healthy volunteers, supporting its advancement into patient studies.
About Bambusa Therapeutics, Inc.
Bambusa Therapeutics is a clinical-stage biotechnology company building a portfolio designed to transform care across a wide spectrum of chronic disease. Powered by an innovative antibody engineering platform enhanced with half-life extension and high-concentration subcutaneous delivery, the company's vision is to deliver transformative medicines for patients across every stage of life, setting the pace for the next era of I&I therapeutics. Headquartered in Boston, Bambusa Therapeutics' pipeline is designed to achieve best-in-disease impact across multiple therapeutic areas:
- BBT001 is a first-in-class half-life extended bispecific antibody targeting IL-4Rα and IL-31, currently in Phase 1 clinical development for atopic dermatitis and other Type 2 inflammatory skin diseases.
- BBT002 is a first-in-class half-life extended bispecific antibody targeting IL-4Rα and IL-5, in Phase 1 clinical development for Type 2 inflammatory disorders including COPD, asthma, and chronic rhinosinusitis with nasal polyps (CRSwNP).
- BBT003 and BBT004 are preclinical programs focused on gastroenterology, including inflammatory bowel disease (IBD), and rheumatological diseases, respectively.
For more information, visit www.bambusatx.com.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Bambusa Therapeutics Announces Highly Positive Healthy Volunteer Results and First Atopic Dermatitis Patient Dosed in Phase I Trial of BBT001
Bambusa Therapeutics Announces Highly Positive Healthy Volunteer Results and First Atopic Dermatitis Patient Dosed in Phase I Trial of BBT001
WUHU, China, Jan. 18, 2026 /PRNewswire/ -- On January 17, the "Technology Meets AI" 2026 Chery AI Night was held in Wuhu, Anhui Province. As the first flagship event of Chery's technology IP "A Thousand and One Nights" in 2026, the event showcased Chery's latest progress in full-scenario AI development. Among the AI innovations presented, AiMOGA Robotics, representing Chery's exploration of embodied intelligence, became one of the key focal points of the evening.
From "Thinking" to "Acting": AiMOGA Robotics as a Key Highlight of the AI Night
As AI moves beyond algorithms and computing power toward deeper integration with the physical world, embodied intelligence is increasingly viewed as a pathway to scalable application. Unlike screen- or voice-based systems, embodied intelligence emphasizes physical presence, autonomous action, and real-time feedback, enabling perception, decision-making, and execution in real environments.
At the AI Night, AiMOGA Robotics demonstrated this transition by extending AI from the digital domain into real-world scenarios. Built on Chery's strengths in intelligent systems and manufacturing integration, AiMOGA is bringing embodied intelligence into everyday life and public service environments.
A Full Robot Family on Display: AiMOGA Showcases a Panorama of Multi-Scenario Applications
A dedicated AiMOGA exhibition and interaction zone was set up at the venue, where the AiMOGA robot family appeared in multiple forms and roles. Exhibits included the traffic police robot "Wuyou" Intelligent Police R001 and the "AiMOGA Care" RN001 medical service robot.
Visitors interacted closely with the robots and observed how embodied intelligence supports communication, guidance, and collaboration in real environments. The AiMOGA robot family became one of the most visited attractions of the event.
AI Beyond Automobiles: AiMOGA Robotics as Chery's "Third Growth Curve"
Within Chery's AI strategy, automobiles remain the core carrier, but not the only one. As one of Chery Group's key third growth curves, AiMOGA Robotics works in synergy with smart mobility, intelligent manufacturing, and the human–vehicle–home ecosystem.
By 2025, AiMOGA had delivered and deployed 300 robots and 1,000 robotic dogs across more than 30 countries and regions. Its humanoid robot Mornine has obtained both hardware and software EU certifications, laying a foundation for global expansion. AiMOGA remains committed to building a trusted human intelligent assistant through practical technology and real-world applications.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

AiMOGA Robot Family Debuts at the 2026 Chery AI Night, Showcasing Diverse Real-World Applications