|
By embedding AI agents and MCP servers into enterprise compliance workflows, Avalara is evolving compliance into a smart, interoperable global framework, that operates at unprecedented scale
DURHAM, N.C., Oct. 7, 2025 /PRNewswire/ -- Avalara, Inc., the agentic tax and compliance leader, today announced breakthroughs in enterprise compliance: Avi, Avalara's AI agent is now embedded directly where users work; cutting-edge Model Context Protocol (MCP) servers provide easy access to Avalara's AI-powered platform; and a series of AI innovations are newly available in its product suite.
These capabilities enable third-party agents and systems to more seamlessly discover and connect with APIs across Avalara's ecosystem, embedding compliance directly into customer workflows and systems. The result is a shift from isolated features to a fully interoperable framework that ensures compliance happens wherever business happens.
"Compliance is no longer a back-office burden, but a mission-critical function," said Scott McFarlane, CEO and Co-Founder of Avalara. "With Avalara's agentic solutions, our customers are cutting filing times from days to hours, reducing compliance risk, and freeing teams to focus on growth."
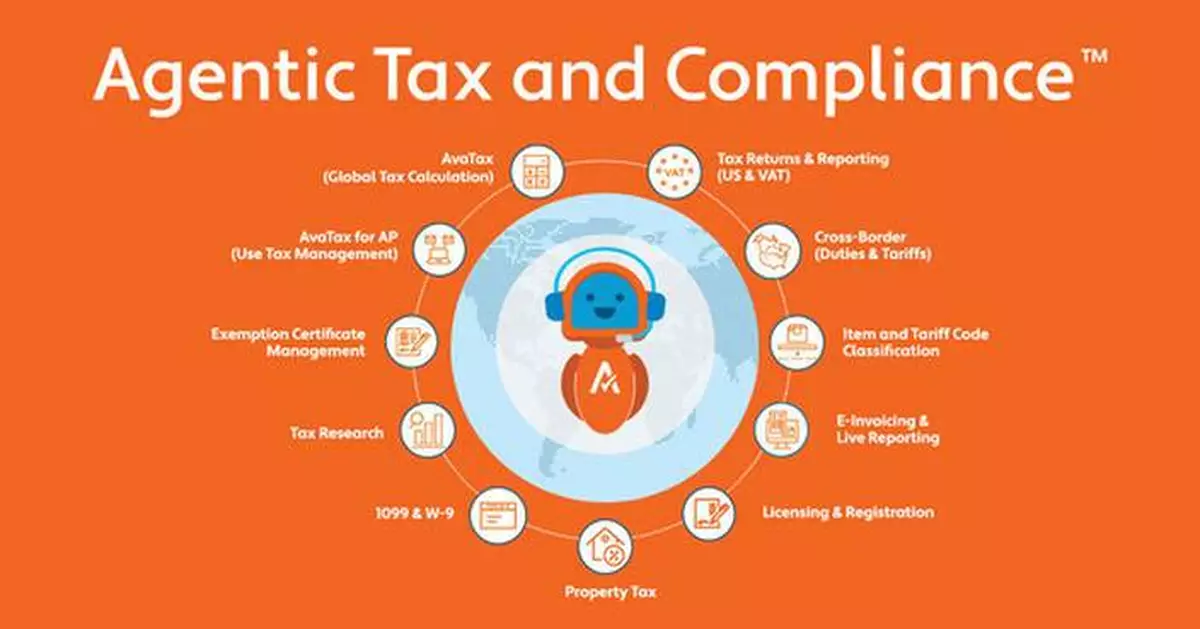
Avi Everywhere, Compliance at the Point of Work Powered by Agents
Avalara's AI agent, Avi, is now embedded directly where users work, observing, advising, and executing compliance for daily workflows:
- Avi Browser Extension: Guides users interactively through ERP configurations and detects compliance actions on the fly (e.g. "do you need an exemption certificate for the vendor you just added?"). It is also there to answer any compliance-related questions users may have with Avalara's industry-leading content.
- Avi in Outlook: Scans inbound invoices, digitizes and validates them for taxes, duties, tariffs, and certificates, then integrates results into ERP systems. This reduces invoice processing costs while catching compliance risks earlier.
MCP Servers: The Backbone of Intelligent Interoperable Compliance
Avalara pioneered cloud-based tax automation and quickly rose to market leadership over the past two decades. Now, as agentic AI reshapes enterprise software, Avalara is once again first to transform the category with an agentic operating model built on MCP servers, enterprise LLMs, domain-specific SLMs, AI agents, and the most expansive compliance content network—all residing in the market's most resilient, scalable, performant, multi-cloud platform.
Third-party agents and systems can connect to Avalara's MCP servers to discover and call APIs across Avalara's platform, enabling applications to connect more seamlessly and extend functionality. By embedding AI agents and MCP servers into enterprise workflows, Avalara is evolving compliance into a smart, interoperable framework, extending trusted category intelligence across global systems at unprecedented scale.
New Enterprise-Grade AI Features
Avalara is introducing a series of new AI-driven capabilities including:
- Advanced Rules with AI Visualization - Users can now create, edit, and visualize compliance rules through natural language prompts. Whether writing an entire rule, tweaking logic, or seeking AI-driven recommendations, compliance teams gain unprecedented precision, speed, and control.
- AI-Powered Reporting and Insights - Avalara's reporting suite has been reinvented with embedded AI. New intelligence surfaces anomalies, highlights trends, and generates custom reports and visualizations—helping finance teams move from reactive reporting to proactive compliance management.
- AI-Driven Global E-Invoicing - Avalara is using AI to auto-map standardized invoice fields to country-specific compliance requirements worldwide. Customers face only minimal customizations, supported by visual mapping tools—accelerating time-to-compliance while helping to ensure greater accuracy across global jurisdictions.
These enterprise-grade capabilities reinforce Avalara's leadership in compliance automation, as recognized in the IDC MarketScape Report: Worldwide SaaS and Cloud-Enabled SaaS Sales and Use Tax Automation Software for Enterprise 2024 Vendor Assessment.
Expanded AI Capabilities
Avalara is also extending its agentic platform across new compliance domains:
- Exemption Certificate Validation – Digitizes and validates certificates with AI, preventing costly audit exposure.
- Cross-Border Classification – Determines harmonized system (HS) codes and flags trade restrictions, solving two of the toughest challenges in global trade.
- Generative Tax Research – Provides compliance-critical answers with authoritative citations, leveraging Avalara's unrivaled tax content for accuracy that eclipses generic AI models.
- Property Tax – Offers AI-driven classification of fixed assets and geography assignment.
"The AI-powered search in Avalara Tax Research has been a game changer, cutting my research time by more than 80 percent," said Dakota Cox, Accounts Payable at Averitt Express, an Avalara customer.
Ecosystem and Market Rollout
Avalara is driving adoption of these innovations through its partner and customer networks:
- Agent-to-Agent (A2A) Registry: Avalara will register Avi in Google AgentSpace and in other registries, making Avalara's tax and compliance capabilities discoverable to other enterprise AI systems, helping to ensure they can be invoked wherever businesses operate.
- MCP Server Registries: Avalara will register its MCP servers in many popular marketplaces and registries for discovery and inclusion in customer agentic workflows.
- Face-to-Face Meetings: Avalara account managers are in the field meeting with partners and VARs, demonstrating new capabilities and educating customers on their impact.
- Avalara Events: The company is hosting events for developers, partners, and customers to gain hands-on access to Avalara's latest offerings.
About Avalara
Avalara is the agentic tax and compliance leader. For more than two decades, Avalara has developed one of the most expansive libraries of tax content and integrations in the industry, supporting over 43,000 businesses and government entities across more than 75 countries. The company's purpose-built AI agents automate end-to-end compliance processes with greater precision, from tax calculations and return filings to exemption certificate management and beyond. For more information, visit Avalara.com.
Photo - https://mma.prnasia.com/media2/2789855/Avi_Everywhere.jpg?p=medium600
Logo - https://mma.prnasia.com/media2/2148367/Avalara_Logo_Tag_RGB_Logo.jpg?p=medium600
By embedding AI agents and MCP servers into enterprise compliance workflows, Avalara is evolving compliance into a smart, interoperable global framework, that operates at unprecedented scale
DURHAM, N.C., Oct. 7, 2025 /PRNewswire/ -- Avalara, Inc., the agentic tax and compliance leader, today announced breakthroughs in enterprise compliance: Avi, Avalara's AI agent is now embedded directly where users work; cutting-edge Model Context Protocol (MCP) servers provide easy access to Avalara's AI-powered platform; and a series of AI innovations are newly available in its product suite.
These capabilities enable third-party agents and systems to more seamlessly discover and connect with APIs across Avalara's ecosystem, embedding compliance directly into customer workflows and systems. The result is a shift from isolated features to a fully interoperable framework that ensures compliance happens wherever business happens.
"Compliance is no longer a back-office burden, but a mission-critical function," said Scott McFarlane, CEO and Co-Founder of Avalara. "With Avalara's agentic solutions, our customers are cutting filing times from days to hours, reducing compliance risk, and freeing teams to focus on growth."
Avi Everywhere, Compliance at the Point of Work Powered by Agents
Avalara's AI agent, Avi, is now embedded directly where users work, observing, advising, and executing compliance for daily workflows:
- Avi Browser Extension: Guides users interactively through ERP configurations and detects compliance actions on the fly (e.g. "do you need an exemption certificate for the vendor you just added?"). It is also there to answer any compliance-related questions users may have with Avalara's industry-leading content.
- Avi in Outlook: Scans inbound invoices, digitizes and validates them for taxes, duties, tariffs, and certificates, then integrates results into ERP systems. This reduces invoice processing costs while catching compliance risks earlier.
MCP Servers: The Backbone of Intelligent Interoperable Compliance
Avalara pioneered cloud-based tax automation and quickly rose to market leadership over the past two decades. Now, as agentic AI reshapes enterprise software, Avalara is once again first to transform the category with an agentic operating model built on MCP servers, enterprise LLMs, domain-specific SLMs, AI agents, and the most expansive compliance content network—all residing in the market's most resilient, scalable, performant, multi-cloud platform.
Third-party agents and systems can connect to Avalara's MCP servers to discover and call APIs across Avalara's platform, enabling applications to connect more seamlessly and extend functionality. By embedding AI agents and MCP servers into enterprise workflows, Avalara is evolving compliance into a smart, interoperable framework, extending trusted category intelligence across global systems at unprecedented scale.
New Enterprise-Grade AI Features
Avalara is introducing a series of new AI-driven capabilities including:
- Advanced Rules with AI Visualization - Users can now create, edit, and visualize compliance rules through natural language prompts. Whether writing an entire rule, tweaking logic, or seeking AI-driven recommendations, compliance teams gain unprecedented precision, speed, and control.
- AI-Powered Reporting and Insights - Avalara's reporting suite has been reinvented with embedded AI. New intelligence surfaces anomalies, highlights trends, and generates custom reports and visualizations—helping finance teams move from reactive reporting to proactive compliance management.
- AI-Driven Global E-Invoicing - Avalara is using AI to auto-map standardized invoice fields to country-specific compliance requirements worldwide. Customers face only minimal customizations, supported by visual mapping tools—accelerating time-to-compliance while helping to ensure greater accuracy across global jurisdictions.
These enterprise-grade capabilities reinforce Avalara's leadership in compliance automation, as recognized in the IDC MarketScape Report: Worldwide SaaS and Cloud-Enabled SaaS Sales and Use Tax Automation Software for Enterprise 2024 Vendor Assessment.
Expanded AI Capabilities
Avalara is also extending its agentic platform across new compliance domains:
- Exemption Certificate Validation – Digitizes and validates certificates with AI, preventing costly audit exposure.
- Cross-Border Classification – Determines harmonized system (HS) codes and flags trade restrictions, solving two of the toughest challenges in global trade.
- Generative Tax Research – Provides compliance-critical answers with authoritative citations, leveraging Avalara's unrivaled tax content for accuracy that eclipses generic AI models.
- Property Tax – Offers AI-driven classification of fixed assets and geography assignment.
"The AI-powered search in Avalara Tax Research has been a game changer, cutting my research time by more than 80 percent," said Dakota Cox, Accounts Payable at Averitt Express, an Avalara customer.
Ecosystem and Market Rollout
Avalara is driving adoption of these innovations through its partner and customer networks:
- Agent-to-Agent (A2A) Registry: Avalara will register Avi in Google AgentSpace and in other registries, making Avalara's tax and compliance capabilities discoverable to other enterprise AI systems, helping to ensure they can be invoked wherever businesses operate.
- MCP Server Registries: Avalara will register its MCP servers in many popular marketplaces and registries for discovery and inclusion in customer agentic workflows.
- Face-to-Face Meetings: Avalara account managers are in the field meeting with partners and VARs, demonstrating new capabilities and educating customers on their impact.
- Avalara Events: The company is hosting events for developers, partners, and customers to gain hands-on access to Avalara's latest offerings.
About Avalara
Avalara is the agentic tax and compliance leader. For more than two decades, Avalara has developed one of the most expansive libraries of tax content and integrations in the industry, supporting over 43,000 businesses and government entities across more than 75 countries. The company's purpose-built AI agents automate end-to-end compliance processes with greater precision, from tax calculations and return filings to exemption certificate management and beyond. For more information, visit Avalara.com.
Photo - https://mma.prnasia.com/media2/2789855/Avi_Everywhere.jpg?p=medium600
Logo - https://mma.prnasia.com/media2/2148367/Avalara_Logo_Tag_RGB_Logo.jpg?p=medium600
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
Avalara Extends AI Leadership with Avi Everywhere, MCP Servers, and Global Compliance Innovations
|
HIGHLIGHTS
- Following an interim data review of the Cohort Expansion Phase (Phase II) of the SECuRE trial, the Safety Review Committee (SRC) confirms the trial will continue with no modifications to the protocol.
Patient population
- With the SECuRE trial continuing to recruit, a total of nine participants who had evaluable data by the 25th of November 2025 were included in the interim assessment by the SRC, with the majority of participants receiving at least two cycles of 8 GBq of 67Cu-SAR-bisPSMA each by the data cut-off date.
- Seven participants received 67Cu-SAR-bisPSMA and two participants were treated with a combination of 67Cu-SAR-bisPSMA with enzalutamide.
- Most participants were heavily pre-treated (with 55.6% having received more than 5 previous anti-cancer regimens) and had bone metastasis (66.7%).
Safety
- The safety profile of 67Cu-SAR-bisPSMA remains favourable with most related adverse events (AEs) in the Cohort Expansion to date being Grade 1 or 2. The most common AEs were nausea and lymphopenia (observed in 33.3% of participants, for each AE).
Efficacy
- Six participants had at least two prostate specific antigen (PSA) results following 67Cu-SAR-bisPSMA administration, with all showing a decrease in PSA. Of those, four participants (66.7%) thus far had a reduction of more than 50% in PSA (PSA50) and two participants (33.3%) had a reduction of more than 80% (PSA80).
- One participant who presented with bone metastasis at enrolment achieved undetectable PSA with no prostate cancer detected by computed tomography (CT) or bone scan following 67Cu-SAR-bisPSMA treatment. The participant reported having excellent quality of life following the treatment.
Next Steps
- The SECuRE trial will continue enrolment into the Cohort Expansion Phase with planned completion of recruitment in 2026. Phase III registrational trial planning ongoing based on data generated to date.
SYDNEY, Jan. 15, 2026 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to share a number of updates on the SECuRE trial following an SRC meeting. The SRC has recommended that the trial continue with the Cohort Expansion Phase (Phase II) as planned with no modifications to the protocol. The interim results assessed by the SRC were collected from nine participants enrolled in the cohort that had evaluable data by the cut-off date of the 25th of November 2025 and continue to show promising efficacy and a favourable safety profile of 67Cu-SAR-bisPSMA.
The majority of the nine participants had bone metastasis at enrolment (66.7%) and received multiple lines of previous treatments (more than 5 previous anti-cancer regimens, 55.6%). Median PSA prior to 67Cu-SAR-bisPSMA treatment was 18.9 ng/mL (range 1.5-30.2 ng/mL). Six out of these nine participants received at least 2 cycles of 8 GBq of 67Cu-SAR-bisPSMA each, with two of them also receiving concomitant enzalutamide.
Of the nine participants included in this SRC analysis, six had at least two PSA results following their 67Cu-SAR-bisPSMA treatment by the data cut-off date. Of these six participants, thus far four (66.7%) showed reductions in PSA of 50% or more (PSA50) and two (33.3%) showed reductions of 80% or more (PSA80).
The safety profile of 67Cu-SAR-bisPSMA remains favourable in the Cohort Expansion, with the majority of related AEs being Grade 1 or 2. The most common related AEs were nausea and lymphopenia (observed in three out of nine participants [33.3%], for each AE). The only AE that was Grade 3 or above was lymphopenia observed in three participants, some of whom had bone metastasis at baseline and/or had received multiple lines of therapy, including taxane and an investigational agent, prior to enrolment in the SECuRE study. There have been no overall renal toxicity or electrocardiogram (ECG) changes observed in these participants. In the combination enzalutamide arm, no new AEs (or worsening of AEs) related to 67Cu-SAR-bisPSMA have been observed to date.
Trial participant with no detectable disease after 3 cycles of 67Cu-SAR-bisPSMA
One of the participants in the Cohort Expansion was a 64-year-old man with bone metastases and baseline PSA of 5.4 ng/mL prior to entering the SECuRE study. Following his first cycle of 67Cu-SAR-bisPSMA, this participant showed a dramatic 95.2% reduction in PSA. He went on to receive 2 more cycles of 67Cu-SAR-bisPSMA and achieved undetectable PSA levels. In a follow-up bone scan and CT no metastatic disease was observed. This participant only exhibited mild (Grade 1) related AEs, most of which were gastrointestinal events, with no haematological or renal AEs observed. The participant reported having excellent quality of life following the treatment.
The interim data from this Phase II continues to confirm the favourable safety profile and promising efficacy seen in previous cohorts of the SECuRE trial[1] and supports the continuation of the trial with the aim to progress to a registrational Phase III study.
Clarity's Executive Chairperson, Dr Alan Taylor, commented, "SAR-bisPSMA continues generating world-class data in both theranostic and diagnostic trials. The combination of the optimised dimer 'bis' structure with the benefits of copper isotopes, enabled by the proprietary sarcophagine technology, is proving to have created a product that is here to challenge the current treatment and diagnostic paradigms in radiopharmaceuticals.
"We have already seen a glimpse of the effects of 67Cu-SAR-bisPSMA through our Dose Escalation cohorts with additional and similar data being generated in the Cohort Expansion phase, demonstrating once again excellent efficacy and safety results of 67Cu-SAR-bisPSMA.
"All of the participants with evaluable data treated in the Phase II to date have shown declines in PSA, with the majority showing PSA decreases of more than 50% and mostly having only mild or moderate AEs. Most of these patients have been treated with more than 5 systemic treatment regiments and had bone metastasis prior to entering the SECuRE study. Although the number of participants with evaluable data to date is small, it is incredible to see yet another extraordinary case where a patient who had bone metastasis prior to entering the study achieved undetectable PSA following 67Cu-SAR-bisPSMA treatment, with no disease observed by anatomical and molecular imaging at the last assessments. This participant only experienced mild, transient AEs, most being gastrointestinal, and has reported having excellent quality of life following the treatment.
"Importantly, the work we have undertaken during the Dose Escalation Phase is now continuing to provide a strong foundation for us as we look ahead at protocol development and dosing for our Phase III clinical trial and commercialisation. As the participant numbers continue to increase with the trial enrollment, we continue to see very promising responses over and over again, giving us more confidence about the future of this product and its potential for commercialisation in metastatic castration-resistant prostate cancer (mCRPC). With three Fast Track Designations for the SAR-bisPSMA product and positive interactions with the US Food and Drug Administration (FDA) to date, we are working towards bringing this agent to clinicians and their patients around the world through the entirety of the prostate cancer journey, from first diagnosis to late-stage disease. All of these indications, being imaging in pre-definitive therapy and biochemical recurrence, as well as therapy in mCRPC, are blockbuster markets individually for prostate-specific membrane antigen (PSMA) targeted products, with an estimated combined market value of approximately US$10-15 billion by 2030. We are committed to continuing the development of this product, aiming to bring improved diagnostic and treatment options for prostate cancer in various stages of their disease."
About the SECuRE trial
The SECuRE trial (NCT04868604)[2] is a Phase I/IIa theranostic trial for identification and treatment of participants with PSMA-expressing mCRPC using 64Cu/67Cu-SAR-bisPSMA. 64Cu-SAR-bisPSMA is used to visualise PSMA-expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation study with a cohort expansion involving approximately 54 participants in the US. The overall aim of the trial is to determine the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of prostate cancer.
The SECuRE trial consists of the Dose Escalation (Phase I) and Cohort Expansion (Phase II) Phases. Based on the data from the Dose Escalation Phase, which demonstrated a favourable safety profile and efficacy of 67Cu-SAR-bisPSMA, the SECuRE trial progressed to the Cohort Expansion (Phase II) at an 8 GBq dose level as per the SRC recommendation (up to 6 cycles per patient in total)[3].
Cohort 2 of the Dose Escalation phase of the trial, where participants were dosed with 8 GBq of 67Cu-SAR-bisPSMA, demonstrated a very low rate of related AEs while all three participants achieved PSA declines of 80% or more (PSA80)[1]. The Dose Escalation Phase also showed high PSA response rates of the mCRPC in the pre-chemotherapy setting with a favourable safety profile: 92% of pre-chemotherapy participants (12/13) demonstrated PSA drops greater than 35%, PSA reductions greater than 50% were reached in 61.5% (8/13) of participants, and reductions of 80% or more were achieved in 46.2% (6/13) of participants[1]. These results supported the progress of the trial to its Cohort Expansion Phase using 8 GBq multi-dose in participants who had not received chemotherapy in the mCRPC setting.
Recruitment is currently ongoing into the Cohort Expansion Phase which will include 24 participants. A subset of participants will be treated with the combination of 8 GBq of 67Cu-SAR-bisPSMA with enzalutamide (androgen receptor pathway inhibitor [ARPI]), in line with the positive results from the Enza-p trial[4] and previous discussions with and advice from key global medical experts in the field of prostate cancer, including the Company's Clinical Advisory Board members, Prof Louise Emmett and Prof Oliver Sartor, as well as the SRC.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis", which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA are unregistered products. The safety and efficacy of 67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA have not been assessed by health authorities such as the US FDA or the Therapeutic Goods Administration (TGA). There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death in men worldwide[5]. Prostate cancer is the second-leading causes of cancer death in American men. The American Cancer Institute estimates in 2025 there will be about 313,780 new cases of prostate cancer in the US and around 35,770 deaths from the disease[6].
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing TCTs based on its SAR Technology Platform for the treatment of cancers.
www.claritypharmaceuticals.com
For more information, please contact:
References
- Clarity Pharmaceuticals. SECuRE trial update: 92% of pre-chemo participants experience greater than 35% drop in PSA levels across all cohorts. Cohort Expansion Phase commences. https://www.claritypharmaceuticals.com/news/secure-update/
- ClinicalTrials.gov Identifier: NCT04868604, https://clinicaltrials.gov/ct2/show/NCT04868604
- Clarity Pharmaceuticals. SECuRE trial update: First patient treated in the Phase II Cohort Expansion. https://www.claritypharmaceuticals.com/news/secure-fp-phase2/
- Emmett L et al. ENZA-p Trial Investigators; Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Overall survival and quality of life with [177Lu]Lu-PSMA-617 plus enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer (ENZA-p): secondary outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2025 Mar;26(3):291-299. doi: 10.1016/S1470-2045(25)00009-9.
- Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21834
- American Cancer Society: Key Statistics for Prostate Cancer. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
This announcement has been authorised for release by the Executive Chairperson.
HIGHLIGHTS
- Following an interim data review of the Cohort Expansion Phase (Phase II) of the SECuRE trial, the Safety Review Committee (SRC) confirms the trial will continue with no modifications to the protocol.
Patient population
- With the SECuRE trial continuing to recruit, a total of nine participants who had evaluable data by the 25th of November 2025 were included in the interim assessment by the SRC, with the majority of participants receiving at least two cycles of 8 GBq of 67Cu-SAR-bisPSMA each by the data cut-off date.
- Seven participants received 67Cu-SAR-bisPSMA and two participants were treated with a combination of 67Cu-SAR-bisPSMA with enzalutamide.
- Most participants were heavily pre-treated (with 55.6% having received more than 5 previous anti-cancer regimens) and had bone metastasis (66.7%).
Safety
- The safety profile of 67Cu-SAR-bisPSMA remains favourable with most related adverse events (AEs) in the Cohort Expansion to date being Grade 1 or 2. The most common AEs were nausea and lymphopenia (observed in 33.3% of participants, for each AE).
Efficacy
- Six participants had at least two prostate specific antigen (PSA) results following 67Cu-SAR-bisPSMA administration, with all showing a decrease in PSA. Of those, four participants (66.7%) thus far had a reduction of more than 50% in PSA (PSA50) and two participants (33.3%) had a reduction of more than 80% (PSA80).
- One participant who presented with bone metastasis at enrolment achieved undetectable PSA with no prostate cancer detected by computed tomography (CT) or bone scan following 67Cu-SAR-bisPSMA treatment. The participant reported having excellent quality of life following the treatment.
Next Steps
- The SECuRE trial will continue enrolment into the Cohort Expansion Phase with planned completion of recruitment in 2026. Phase III registrational trial planning ongoing based on data generated to date.
SYDNEY, Jan. 15, 2026 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to share a number of updates on the SECuRE trial following an SRC meeting. The SRC has recommended that the trial continue with the Cohort Expansion Phase (Phase II) as planned with no modifications to the protocol. The interim results assessed by the SRC were collected from nine participants enrolled in the cohort that had evaluable data by the cut-off date of the 25th of November 2025 and continue to show promising efficacy and a favourable safety profile of 67Cu-SAR-bisPSMA.
The majority of the nine participants had bone metastasis at enrolment (66.7%) and received multiple lines of previous treatments (more than 5 previous anti-cancer regimens, 55.6%). Median PSA prior to 67Cu-SAR-bisPSMA treatment was 18.9 ng/mL (range 1.5-30.2 ng/mL). Six out of these nine participants received at least 2 cycles of 8 GBq of 67Cu-SAR-bisPSMA each, with two of them also receiving concomitant enzalutamide.
Of the nine participants included in this SRC analysis, six had at least two PSA results following their 67Cu-SAR-bisPSMA treatment by the data cut-off date. Of these six participants, thus far four (66.7%) showed reductions in PSA of 50% or more (PSA50) and two (33.3%) showed reductions of 80% or more (PSA80).
The safety profile of 67Cu-SAR-bisPSMA remains favourable in the Cohort Expansion, with the majority of related AEs being Grade 1 or 2. The most common related AEs were nausea and lymphopenia (observed in three out of nine participants [33.3%], for each AE). The only AE that was Grade 3 or above was lymphopenia observed in three participants, some of whom had bone metastasis at baseline and/or had received multiple lines of therapy, including taxane and an investigational agent, prior to enrolment in the SECuRE study. There have been no overall renal toxicity or electrocardiogram (ECG) changes observed in these participants. In the combination enzalutamide arm, no new AEs (or worsening of AEs) related to 67Cu-SAR-bisPSMA have been observed to date.
Trial participant with no detectable disease after 3 cycles of 67Cu-SAR-bisPSMA
One of the participants in the Cohort Expansion was a 64-year-old man with bone metastases and baseline PSA of 5.4 ng/mL prior to entering the SECuRE study. Following his first cycle of 67Cu-SAR-bisPSMA, this participant showed a dramatic 95.2% reduction in PSA. He went on to receive 2 more cycles of 67Cu-SAR-bisPSMA and achieved undetectable PSA levels. In a follow-up bone scan and CT no metastatic disease was observed. This participant only exhibited mild (Grade 1) related AEs, most of which were gastrointestinal events, with no haematological or renal AEs observed. The participant reported having excellent quality of life following the treatment.
The interim data from this Phase II continues to confirm the favourable safety profile and promising efficacy seen in previous cohorts of the SECuRE trial[1] and supports the continuation of the trial with the aim to progress to a registrational Phase III study.
Clarity's Executive Chairperson, Dr Alan Taylor, commented, "SAR-bisPSMA continues generating world-class data in both theranostic and diagnostic trials. The combination of the optimised dimer 'bis' structure with the benefits of copper isotopes, enabled by the proprietary sarcophagine technology, is proving to have created a product that is here to challenge the current treatment and diagnostic paradigms in radiopharmaceuticals.
"We have already seen a glimpse of the effects of 67Cu-SAR-bisPSMA through our Dose Escalation cohorts with additional and similar data being generated in the Cohort Expansion phase, demonstrating once again excellent efficacy and safety results of 67Cu-SAR-bisPSMA.
"All of the participants with evaluable data treated in the Phase II to date have shown declines in PSA, with the majority showing PSA decreases of more than 50% and mostly having only mild or moderate AEs. Most of these patients have been treated with more than 5 systemic treatment regiments and had bone metastasis prior to entering the SECuRE study. Although the number of participants with evaluable data to date is small, it is incredible to see yet another extraordinary case where a patient who had bone metastasis prior to entering the study achieved undetectable PSA following 67Cu-SAR-bisPSMA treatment, with no disease observed by anatomical and molecular imaging at the last assessments. This participant only experienced mild, transient AEs, most being gastrointestinal, and has reported having excellent quality of life following the treatment.
"Importantly, the work we have undertaken during the Dose Escalation Phase is now continuing to provide a strong foundation for us as we look ahead at protocol development and dosing for our Phase III clinical trial and commercialisation. As the participant numbers continue to increase with the trial enrollment, we continue to see very promising responses over and over again, giving us more confidence about the future of this product and its potential for commercialisation in metastatic castration-resistant prostate cancer (mCRPC). With three Fast Track Designations for the SAR-bisPSMA product and positive interactions with the US Food and Drug Administration (FDA) to date, we are working towards bringing this agent to clinicians and their patients around the world through the entirety of the prostate cancer journey, from first diagnosis to late-stage disease. All of these indications, being imaging in pre-definitive therapy and biochemical recurrence, as well as therapy in mCRPC, are blockbuster markets individually for prostate-specific membrane antigen (PSMA) targeted products, with an estimated combined market value of approximately US$10-15 billion by 2030. We are committed to continuing the development of this product, aiming to bring improved diagnostic and treatment options for prostate cancer in various stages of their disease."
About the SECuRE trial
The SECuRE trial (NCT04868604)[2] is a Phase I/IIa theranostic trial for identification and treatment of participants with PSMA-expressing mCRPC using 64Cu/67Cu-SAR-bisPSMA. 64Cu-SAR-bisPSMA is used to visualise PSMA-expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation study with a cohort expansion involving approximately 54 participants in the US. The overall aim of the trial is to determine the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of prostate cancer.
The SECuRE trial consists of the Dose Escalation (Phase I) and Cohort Expansion (Phase II) Phases. Based on the data from the Dose Escalation Phase, which demonstrated a favourable safety profile and efficacy of 67Cu-SAR-bisPSMA, the SECuRE trial progressed to the Cohort Expansion (Phase II) at an 8 GBq dose level as per the SRC recommendation (up to 6 cycles per patient in total)[3].
Cohort 2 of the Dose Escalation phase of the trial, where participants were dosed with 8 GBq of 67Cu-SAR-bisPSMA, demonstrated a very low rate of related AEs while all three participants achieved PSA declines of 80% or more (PSA80)[1]. The Dose Escalation Phase also showed high PSA response rates of the mCRPC in the pre-chemotherapy setting with a favourable safety profile: 92% of pre-chemotherapy participants (12/13) demonstrated PSA drops greater than 35%, PSA reductions greater than 50% were reached in 61.5% (8/13) of participants, and reductions of 80% or more were achieved in 46.2% (6/13) of participants[1]. These results supported the progress of the trial to its Cohort Expansion Phase using 8 GBq multi-dose in participants who had not received chemotherapy in the mCRPC setting.
Recruitment is currently ongoing into the Cohort Expansion Phase which will include 24 participants. A subset of participants will be treated with the combination of 8 GBq of 67Cu-SAR-bisPSMA with enzalutamide (androgen receptor pathway inhibitor [ARPI]), in line with the positive results from the Enza-p trial[4] and previous discussions with and advice from key global medical experts in the field of prostate cancer, including the Company's Clinical Advisory Board members, Prof Louise Emmett and Prof Oliver Sartor, as well as the SRC.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis", which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA are unregistered products. The safety and efficacy of 67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA have not been assessed by health authorities such as the US FDA or the Therapeutic Goods Administration (TGA). There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death in men worldwide[5]. Prostate cancer is the second-leading causes of cancer death in American men. The American Cancer Institute estimates in 2025 there will be about 313,780 new cases of prostate cancer in the US and around 35,770 deaths from the disease[6].
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing TCTs based on its SAR Technology Platform for the treatment of cancers.
www.claritypharmaceuticals.com
For more information, please contact:
Clarity Pharmaceuticals
Dr Alan Taylor
Lisa Sadetskaya
Executive Chairperson
Director, Corporate Communications
ataylor@claritypharm.com
lisa@claritypharm.com
References
This announcement has been authorised for release by the Executive Chairperson.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

SECuRE trial to continue with no modifications to protocol following Safety Review Committee meeting