SHANGHAI, Oct. 15, 2025 /PRNewswire/ -- vivo today announced the global launch of vivo OriginOS, the company's most advanced operating system, redefining human-computer interaction in the AI era.
"For years, vivo has deepened its global presence under the principle of 'More Local, More Global,' and continues to create products and experiences that stay true to users' needs," said SHI Yujian, Senior Vice President and Chief Technology Officer at vivo. "As we celebrate vivo's 30th anniversary, we are taking the next step with the global debut of OriginOS. Guided by our 'Origin Design' philosophy and built on three core pillars of smoothness, design and AI, the brand new OriginOS 6 reimagines the way people connect with the digital world."
Click to Gallery
vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era
vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era
vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era
vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era
vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era
Smoothness Mastery: Look Smooth, Feel Smooth, Stay Smooth
Smoothness lies at the heart of OriginOS 6— more than a feature, it represents a new standard of mastery. The new Origin Smooth Engine enables seamless collaboration among the system's core modules, computing, storage, and display, and delivers an all-around experience that looks smooth, feels smooth, and stays smooth over time.
The 8+1 Ultra-Core Computing keeps critical tasks running first, improving app cold-start speed by 18.5% and frame-rate stability by 10.5%. Memory Fusion accelerates data loading by 106%, while Dual Rendering boosts animation performance by 35% and keeps frame rates 11% steadier under heavy loads[1].
Smoothness is first seen on screen through the Origin Animation system, which enhances the visual experience with motion effects such as Spring Animation, Blur Transition, Morphing Animation, and One Shot Animation, giving each touch a natural rhythm and visual coherence.
It's also smooth to the touch. vivo's industry-first Snap-Up Engine prioritizes computing power for high-demand actions such as ticketing, keeping users one step ahead. Paired with faster app launches, up to 16% quicker when opening 50 consecutive apps, and a refined touch response that's 41% faster, every tap and swipe feels effortlessly smooth.
Beyond the moment, smoothness endures. The latest vivo X300 Pro with OriginOS 6 has earned SGS certification for sustained smoothness, simulating five years of heavy use. Together, these innovations define vivo's mastery of smoothness—an experience users can see, feel, and rely on every day.
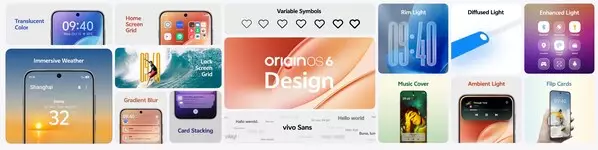
Intuitive Design Inspired by Nature
Inspired by nature and grounded in how people experience the world, OriginOS 6 is designed to make digital life more intuitive, effortless, and delightful.
The Origin Design system unifies visual elements including color, shape, icon, image, font, layout, material and depth, with simplicity and precision. The new brand font, vivo Sans[2], supports over 40 languages, and the symbols now feature fully variable and adjustable weight for the first time. With Dynamic Glow and Translucent Color, light flows across materials, adding depth and immersive vibrancy to every interaction.
Bringing life's sensibilities into the digital world, vivo has also reimagined app design. From checking device status in iManager to tracking well-being in Origin Health, users can focus on ongoing tasks and get things done with ease. The new Immersive Weather further allows users to explore weather in an innovative digital form.
OriginOS 6 also inspires personal expression. The new Lock Screen Grid allows users to arrange and resize widgets freely, customize fonts, and combine photos for instant updates. A reimagined Home Screen Grid introduces a sleek 4×7 layout and adaptive folders, and the Flip Cards bring everyday photos to life with dynamic, living display that shifts naturally as you tilt the device.
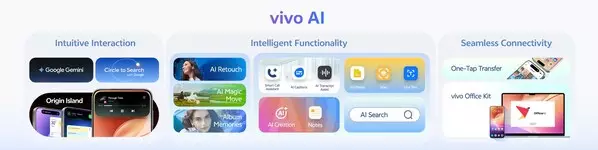
vivo AI Empowering A New Personal Intelligence Experience
vivo AI propels OriginOS 6 into a new chapter of personal intelligence. In close collaboration with Google, it brings the upgraded Gemini and Circle to Search, delivering a smarter and more intuitive experience.
Further, as a new, natural and intuitive form of interaction, the new Origin Island surfaces real-time status at the top of the screen and remains visible when you leave apps, integrated with Android 16's Live Updates integration. It offers contextual suggestions you can act on instantly, enabling Copy & Go—copy a phone number to call, text or save as a new contact, or copy meeting details to create a calendar item, and Drag & Go—edit a photo, for example, and drag it to Origin Island to continue in the suggested app without switching.
vivo AI transforms both creativity and productivity across everyday scenarios. In imaging capabilities, the new AI Retouch integrates AI Erase, AI Image Expander, and AI Photo Enhance into a single, seamless workflow for effortless enhancement. In everyday productivity, vivo AI empowers communication, document handling, content creation, and information search through intelligent tools such as Smart Call Assistant, DocMaster, AI Creation, and AI Search—making daily work simpler and more efficient.
Extending beyond a single device, vivo AI enables seamless collaboration through features such as the Office Kit[3] and One-Tap Transfer[4], to keep work and creativity flowing effortlessly across devices and platforms.
Security and Endurance to Rely On
OriginOS 6 strengthens protection with the vivo Security brand to safeguard data security and privacy, ensuring transparency and control, on-device intelligence, and data minimization. The upgraded BlueVolt technology further improves power efficiency and charging stability, keeping performance cool, stable and long-lasting.
Availability
OriginOS 6 will begin rolling out globally in phases starting from November 2025. Further upgrade plan may vary by markets.
(END)
About vivo
vivo is a technology company that creates great products based on a design-driven value, with smart devices and intelligent services as its core. The company aims to build a bridge between humans and the digital world. Through unique creativity, vivo provides users with an increasingly convenient mobile and digital life. Following the company's core values, which include Benfen*, user-orientation, design-driven value, continuous learning, and team spirit, vivo has implemented a sustainable development strategy with the vision of developing into a healthier, more sustainable world-class corporation.
While bringing together and developing the best local talents to deliver excellence, vivo is supported by a network of R&D centers in Shenzhen, Dongguan, Nanjing, Beijing, Hangzhou, Shanghai, Xi'an and more cities, focusing on the development of state-of-the-art consumer technologies, including 5G, artificial intelligence, industrial design, imaging system and other up-and-coming technologies. vivo has also set up an intelligent manufacturing network (including those authorized by vivo), with an annual production capacity of nearly 200 million smartphones. As of now, vivo has branched out its sales network across more than 60 countries and regions and is loved by more than 500 million users worldwide.
*"Benfen" is a term describing the attitude on doing the right things and doing things right – which is the ideal description of vivo's mission to create value for society.
Stay informed of latest vivo news at https://www.vivo.com/en/about-vivo/news
| [1] Data was obtained from vivo labs and may vary by model. Actual performance shall prevail. |
| [2] The font name may vary by model and region. Please refer to actual usage. |
| [3] To use Office Kit, install the latest version and get more information from pc.vivoglobal.com. This feature requires third-party service support, which may change. |
| [4] This feature requires third-party service support, which may change. |
[1] Data was obtained from vivo labs and may vary by model. Actual performance shall prevail.
[2] The font name may vary by model and region. Please refer to actual usage.
[3] To use Office Kit, install the latest version and get more information from pc.vivoglobal.com. This feature requires third-party service support, which may change.
[4] This feature requires third-party service support, which may change.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era
vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era

vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era

vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era

vivo Unveils OriginOS Globally, Defining Smoothness Mastery for the Next Mobile Era
|
HIGHLIGHTS
- Following an interim data review of the Cohort Expansion Phase (Phase II) of the SECuRE trial, the Safety Review Committee (SRC) confirms the trial will continue with no modifications to the protocol.
Patient population
- With the SECuRE trial continuing to recruit, a total of nine participants who had evaluable data by the 25th of November 2025 were included in the interim assessment by the SRC, with the majority of participants receiving at least two cycles of 8 GBq of 67Cu-SAR-bisPSMA each by the data cut-off date.
- Seven participants received 67Cu-SAR-bisPSMA and two participants were treated with a combination of 67Cu-SAR-bisPSMA with enzalutamide.
- Most participants were heavily pre-treated (with 55.6% having received more than 5 previous anti-cancer regimens) and had bone metastasis (66.7%).
Safety
- The safety profile of 67Cu-SAR-bisPSMA remains favourable with most related adverse events (AEs) in the Cohort Expansion to date being Grade 1 or 2. The most common AEs were nausea and lymphopenia (observed in 33.3% of participants, for each AE).
Efficacy
- Six participants had at least two prostate specific antigen (PSA) results following 67Cu-SAR-bisPSMA administration, with all showing a decrease in PSA. Of those, four participants (66.7%) thus far had a reduction of more than 50% in PSA (PSA50) and two participants (33.3%) had a reduction of more than 80% (PSA80).
- One participant who presented with bone metastasis at enrolment achieved undetectable PSA with no prostate cancer detected by computed tomography (CT) or bone scan following 67Cu-SAR-bisPSMA treatment. The participant reported having excellent quality of life following the treatment.
Next Steps
- The SECuRE trial will continue enrolment into the Cohort Expansion Phase with planned completion of recruitment in 2026. Phase III registrational trial planning ongoing based on data generated to date.
SYDNEY, Jan. 15, 2026 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to share a number of updates on the SECuRE trial following an SRC meeting. The SRC has recommended that the trial continue with the Cohort Expansion Phase (Phase II) as planned with no modifications to the protocol. The interim results assessed by the SRC were collected from nine participants enrolled in the cohort that had evaluable data by the cut-off date of the 25th of November 2025 and continue to show promising efficacy and a favourable safety profile of 67Cu-SAR-bisPSMA.
The majority of the nine participants had bone metastasis at enrolment (66.7%) and received multiple lines of previous treatments (more than 5 previous anti-cancer regimens, 55.6%). Median PSA prior to 67Cu-SAR-bisPSMA treatment was 18.9 ng/mL (range 1.5-30.2 ng/mL). Six out of these nine participants received at least 2 cycles of 8 GBq of 67Cu-SAR-bisPSMA each, with two of them also receiving concomitant enzalutamide.
Of the nine participants included in this SRC analysis, six had at least two PSA results following their 67Cu-SAR-bisPSMA treatment by the data cut-off date. Of these six participants, thus far four (66.7%) showed reductions in PSA of 50% or more (PSA50) and two (33.3%) showed reductions of 80% or more (PSA80).
The safety profile of 67Cu-SAR-bisPSMA remains favourable in the Cohort Expansion, with the majority of related AEs being Grade 1 or 2. The most common related AEs were nausea and lymphopenia (observed in three out of nine participants [33.3%], for each AE). The only AE that was Grade 3 or above was lymphopenia observed in three participants, some of whom had bone metastasis at baseline and/or had received multiple lines of therapy, including taxane and an investigational agent, prior to enrolment in the SECuRE study. There have been no overall renal toxicity or electrocardiogram (ECG) changes observed in these participants. In the combination enzalutamide arm, no new AEs (or worsening of AEs) related to 67Cu-SAR-bisPSMA have been observed to date.
Trial participant with no detectable disease after 3 cycles of 67Cu-SAR-bisPSMA
One of the participants in the Cohort Expansion was a 64-year-old man with bone metastases and baseline PSA of 5.4 ng/mL prior to entering the SECuRE study. Following his first cycle of 67Cu-SAR-bisPSMA, this participant showed a dramatic 95.2% reduction in PSA. He went on to receive 2 more cycles of 67Cu-SAR-bisPSMA and achieved undetectable PSA levels. In a follow-up bone scan and CT no metastatic disease was observed. This participant only exhibited mild (Grade 1) related AEs, most of which were gastrointestinal events, with no haematological or renal AEs observed. The participant reported having excellent quality of life following the treatment.
The interim data from this Phase II continues to confirm the favourable safety profile and promising efficacy seen in previous cohorts of the SECuRE trial[1] and supports the continuation of the trial with the aim to progress to a registrational Phase III study.
Clarity's Executive Chairperson, Dr Alan Taylor, commented, "SAR-bisPSMA continues generating world-class data in both theranostic and diagnostic trials. The combination of the optimised dimer 'bis' structure with the benefits of copper isotopes, enabled by the proprietary sarcophagine technology, is proving to have created a product that is here to challenge the current treatment and diagnostic paradigms in radiopharmaceuticals.
"We have already seen a glimpse of the effects of 67Cu-SAR-bisPSMA through our Dose Escalation cohorts with additional and similar data being generated in the Cohort Expansion phase, demonstrating once again excellent efficacy and safety results of 67Cu-SAR-bisPSMA.
"All of the participants with evaluable data treated in the Phase II to date have shown declines in PSA, with the majority showing PSA decreases of more than 50% and mostly having only mild or moderate AEs. Most of these patients have been treated with more than 5 systemic treatment regiments and had bone metastasis prior to entering the SECuRE study. Although the number of participants with evaluable data to date is small, it is incredible to see yet another extraordinary case where a patient who had bone metastasis prior to entering the study achieved undetectable PSA following 67Cu-SAR-bisPSMA treatment, with no disease observed by anatomical and molecular imaging at the last assessments. This participant only experienced mild, transient AEs, most being gastrointestinal, and has reported having excellent quality of life following the treatment.
"Importantly, the work we have undertaken during the Dose Escalation Phase is now continuing to provide a strong foundation for us as we look ahead at protocol development and dosing for our Phase III clinical trial and commercialisation. As the participant numbers continue to increase with the trial enrollment, we continue to see very promising responses over and over again, giving us more confidence about the future of this product and its potential for commercialisation in metastatic castration-resistant prostate cancer (mCRPC). With three Fast Track Designations for the SAR-bisPSMA product and positive interactions with the US Food and Drug Administration (FDA) to date, we are working towards bringing this agent to clinicians and their patients around the world through the entirety of the prostate cancer journey, from first diagnosis to late-stage disease. All of these indications, being imaging in pre-definitive therapy and biochemical recurrence, as well as therapy in mCRPC, are blockbuster markets individually for prostate-specific membrane antigen (PSMA) targeted products, with an estimated combined market value of approximately US$10-15 billion by 2030. We are committed to continuing the development of this product, aiming to bring improved diagnostic and treatment options for prostate cancer in various stages of their disease."
About the SECuRE trial
The SECuRE trial (NCT04868604)[2] is a Phase I/IIa theranostic trial for identification and treatment of participants with PSMA-expressing mCRPC using 64Cu/67Cu-SAR-bisPSMA. 64Cu-SAR-bisPSMA is used to visualise PSMA-expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation study with a cohort expansion involving approximately 54 participants in the US. The overall aim of the trial is to determine the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of prostate cancer.
The SECuRE trial consists of the Dose Escalation (Phase I) and Cohort Expansion (Phase II) Phases. Based on the data from the Dose Escalation Phase, which demonstrated a favourable safety profile and efficacy of 67Cu-SAR-bisPSMA, the SECuRE trial progressed to the Cohort Expansion (Phase II) at an 8 GBq dose level as per the SRC recommendation (up to 6 cycles per patient in total)[3].
Cohort 2 of the Dose Escalation phase of the trial, where participants were dosed with 8 GBq of 67Cu-SAR-bisPSMA, demonstrated a very low rate of related AEs while all three participants achieved PSA declines of 80% or more (PSA80)[1]. The Dose Escalation Phase also showed high PSA response rates of the mCRPC in the pre-chemotherapy setting with a favourable safety profile: 92% of pre-chemotherapy participants (12/13) demonstrated PSA drops greater than 35%, PSA reductions greater than 50% were reached in 61.5% (8/13) of participants, and reductions of 80% or more were achieved in 46.2% (6/13) of participants[1]. These results supported the progress of the trial to its Cohort Expansion Phase using 8 GBq multi-dose in participants who had not received chemotherapy in the mCRPC setting.
Recruitment is currently ongoing into the Cohort Expansion Phase which will include 24 participants. A subset of participants will be treated with the combination of 8 GBq of 67Cu-SAR-bisPSMA with enzalutamide (androgen receptor pathway inhibitor [ARPI]), in line with the positive results from the Enza-p trial[4] and previous discussions with and advice from key global medical experts in the field of prostate cancer, including the Company's Clinical Advisory Board members, Prof Louise Emmett and Prof Oliver Sartor, as well as the SRC.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis", which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA are unregistered products. The safety and efficacy of 67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA have not been assessed by health authorities such as the US FDA or the Therapeutic Goods Administration (TGA). There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death in men worldwide[5]. Prostate cancer is the second-leading causes of cancer death in American men. The American Cancer Institute estimates in 2025 there will be about 313,780 new cases of prostate cancer in the US and around 35,770 deaths from the disease[6].
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing TCTs based on its SAR Technology Platform for the treatment of cancers.
www.claritypharmaceuticals.com
For more information, please contact:
References
- Clarity Pharmaceuticals. SECuRE trial update: 92% of pre-chemo participants experience greater than 35% drop in PSA levels across all cohorts. Cohort Expansion Phase commences. https://www.claritypharmaceuticals.com/news/secure-update/
- ClinicalTrials.gov Identifier: NCT04868604, https://clinicaltrials.gov/ct2/show/NCT04868604
- Clarity Pharmaceuticals. SECuRE trial update: First patient treated in the Phase II Cohort Expansion. https://www.claritypharmaceuticals.com/news/secure-fp-phase2/
- Emmett L et al. ENZA-p Trial Investigators; Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Overall survival and quality of life with [177Lu]Lu-PSMA-617 plus enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer (ENZA-p): secondary outcomes from a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2025 Mar;26(3):291-299. doi: 10.1016/S1470-2045(25)00009-9.
- Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21834
- American Cancer Society: Key Statistics for Prostate Cancer. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
This announcement has been authorised for release by the Executive Chairperson.
HIGHLIGHTS
- Following an interim data review of the Cohort Expansion Phase (Phase II) of the SECuRE trial, the Safety Review Committee (SRC) confirms the trial will continue with no modifications to the protocol.
Patient population
- With the SECuRE trial continuing to recruit, a total of nine participants who had evaluable data by the 25th of November 2025 were included in the interim assessment by the SRC, with the majority of participants receiving at least two cycles of 8 GBq of 67Cu-SAR-bisPSMA each by the data cut-off date.
- Seven participants received 67Cu-SAR-bisPSMA and two participants were treated with a combination of 67Cu-SAR-bisPSMA with enzalutamide.
- Most participants were heavily pre-treated (with 55.6% having received more than 5 previous anti-cancer regimens) and had bone metastasis (66.7%).
Safety
- The safety profile of 67Cu-SAR-bisPSMA remains favourable with most related adverse events (AEs) in the Cohort Expansion to date being Grade 1 or 2. The most common AEs were nausea and lymphopenia (observed in 33.3% of participants, for each AE).
Efficacy
- Six participants had at least two prostate specific antigen (PSA) results following 67Cu-SAR-bisPSMA administration, with all showing a decrease in PSA. Of those, four participants (66.7%) thus far had a reduction of more than 50% in PSA (PSA50) and two participants (33.3%) had a reduction of more than 80% (PSA80).
- One participant who presented with bone metastasis at enrolment achieved undetectable PSA with no prostate cancer detected by computed tomography (CT) or bone scan following 67Cu-SAR-bisPSMA treatment. The participant reported having excellent quality of life following the treatment.
Next Steps
- The SECuRE trial will continue enrolment into the Cohort Expansion Phase with planned completion of recruitment in 2026. Phase III registrational trial planning ongoing based on data generated to date.
SYDNEY, Jan. 15, 2026 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to share a number of updates on the SECuRE trial following an SRC meeting. The SRC has recommended that the trial continue with the Cohort Expansion Phase (Phase II) as planned with no modifications to the protocol. The interim results assessed by the SRC were collected from nine participants enrolled in the cohort that had evaluable data by the cut-off date of the 25th of November 2025 and continue to show promising efficacy and a favourable safety profile of 67Cu-SAR-bisPSMA.
The majority of the nine participants had bone metastasis at enrolment (66.7%) and received multiple lines of previous treatments (more than 5 previous anti-cancer regimens, 55.6%). Median PSA prior to 67Cu-SAR-bisPSMA treatment was 18.9 ng/mL (range 1.5-30.2 ng/mL). Six out of these nine participants received at least 2 cycles of 8 GBq of 67Cu-SAR-bisPSMA each, with two of them also receiving concomitant enzalutamide.
Of the nine participants included in this SRC analysis, six had at least two PSA results following their 67Cu-SAR-bisPSMA treatment by the data cut-off date. Of these six participants, thus far four (66.7%) showed reductions in PSA of 50% or more (PSA50) and two (33.3%) showed reductions of 80% or more (PSA80).
The safety profile of 67Cu-SAR-bisPSMA remains favourable in the Cohort Expansion, with the majority of related AEs being Grade 1 or 2. The most common related AEs were nausea and lymphopenia (observed in three out of nine participants [33.3%], for each AE). The only AE that was Grade 3 or above was lymphopenia observed in three participants, some of whom had bone metastasis at baseline and/or had received multiple lines of therapy, including taxane and an investigational agent, prior to enrolment in the SECuRE study. There have been no overall renal toxicity or electrocardiogram (ECG) changes observed in these participants. In the combination enzalutamide arm, no new AEs (or worsening of AEs) related to 67Cu-SAR-bisPSMA have been observed to date.
Trial participant with no detectable disease after 3 cycles of 67Cu-SAR-bisPSMA
One of the participants in the Cohort Expansion was a 64-year-old man with bone metastases and baseline PSA of 5.4 ng/mL prior to entering the SECuRE study. Following his first cycle of 67Cu-SAR-bisPSMA, this participant showed a dramatic 95.2% reduction in PSA. He went on to receive 2 more cycles of 67Cu-SAR-bisPSMA and achieved undetectable PSA levels. In a follow-up bone scan and CT no metastatic disease was observed. This participant only exhibited mild (Grade 1) related AEs, most of which were gastrointestinal events, with no haematological or renal AEs observed. The participant reported having excellent quality of life following the treatment.
The interim data from this Phase II continues to confirm the favourable safety profile and promising efficacy seen in previous cohorts of the SECuRE trial[1] and supports the continuation of the trial with the aim to progress to a registrational Phase III study.
Clarity's Executive Chairperson, Dr Alan Taylor, commented, "SAR-bisPSMA continues generating world-class data in both theranostic and diagnostic trials. The combination of the optimised dimer 'bis' structure with the benefits of copper isotopes, enabled by the proprietary sarcophagine technology, is proving to have created a product that is here to challenge the current treatment and diagnostic paradigms in radiopharmaceuticals.
"We have already seen a glimpse of the effects of 67Cu-SAR-bisPSMA through our Dose Escalation cohorts with additional and similar data being generated in the Cohort Expansion phase, demonstrating once again excellent efficacy and safety results of 67Cu-SAR-bisPSMA.
"All of the participants with evaluable data treated in the Phase II to date have shown declines in PSA, with the majority showing PSA decreases of more than 50% and mostly having only mild or moderate AEs. Most of these patients have been treated with more than 5 systemic treatment regiments and had bone metastasis prior to entering the SECuRE study. Although the number of participants with evaluable data to date is small, it is incredible to see yet another extraordinary case where a patient who had bone metastasis prior to entering the study achieved undetectable PSA following 67Cu-SAR-bisPSMA treatment, with no disease observed by anatomical and molecular imaging at the last assessments. This participant only experienced mild, transient AEs, most being gastrointestinal, and has reported having excellent quality of life following the treatment.
"Importantly, the work we have undertaken during the Dose Escalation Phase is now continuing to provide a strong foundation for us as we look ahead at protocol development and dosing for our Phase III clinical trial and commercialisation. As the participant numbers continue to increase with the trial enrollment, we continue to see very promising responses over and over again, giving us more confidence about the future of this product and its potential for commercialisation in metastatic castration-resistant prostate cancer (mCRPC). With three Fast Track Designations for the SAR-bisPSMA product and positive interactions with the US Food and Drug Administration (FDA) to date, we are working towards bringing this agent to clinicians and their patients around the world through the entirety of the prostate cancer journey, from first diagnosis to late-stage disease. All of these indications, being imaging in pre-definitive therapy and biochemical recurrence, as well as therapy in mCRPC, are blockbuster markets individually for prostate-specific membrane antigen (PSMA) targeted products, with an estimated combined market value of approximately US$10-15 billion by 2030. We are committed to continuing the development of this product, aiming to bring improved diagnostic and treatment options for prostate cancer in various stages of their disease."
About the SECuRE trial
The SECuRE trial (NCT04868604)[2] is a Phase I/IIa theranostic trial for identification and treatment of participants with PSMA-expressing mCRPC using 64Cu/67Cu-SAR-bisPSMA. 64Cu-SAR-bisPSMA is used to visualise PSMA-expressing lesions and select candidates for subsequent 67Cu-SAR-bisPSMA therapy. The trial is a multi-centre, single arm, dose escalation study with a cohort expansion involving approximately 54 participants in the US. The overall aim of the trial is to determine the safety and efficacy of 67Cu-SAR-bisPSMA for the treatment of prostate cancer.
The SECuRE trial consists of the Dose Escalation (Phase I) and Cohort Expansion (Phase II) Phases. Based on the data from the Dose Escalation Phase, which demonstrated a favourable safety profile and efficacy of 67Cu-SAR-bisPSMA, the SECuRE trial progressed to the Cohort Expansion (Phase II) at an 8 GBq dose level as per the SRC recommendation (up to 6 cycles per patient in total)[3].
Cohort 2 of the Dose Escalation phase of the trial, where participants were dosed with 8 GBq of 67Cu-SAR-bisPSMA, demonstrated a very low rate of related AEs while all three participants achieved PSA declines of 80% or more (PSA80)[1]. The Dose Escalation Phase also showed high PSA response rates of the mCRPC in the pre-chemotherapy setting with a favourable safety profile: 92% of pre-chemotherapy participants (12/13) demonstrated PSA drops greater than 35%, PSA reductions greater than 50% were reached in 61.5% (8/13) of participants, and reductions of 80% or more were achieved in 46.2% (6/13) of participants[1]. These results supported the progress of the trial to its Cohort Expansion Phase using 8 GBq multi-dose in participants who had not received chemotherapy in the mCRPC setting.
Recruitment is currently ongoing into the Cohort Expansion Phase which will include 24 participants. A subset of participants will be treated with the combination of 8 GBq of 67Cu-SAR-bisPSMA with enzalutamide (androgen receptor pathway inhibitor [ARPI]), in line with the positive results from the Enza-p trial[4] and previous discussions with and advice from key global medical experts in the field of prostate cancer, including the Company's Clinical Advisory Board members, Prof Louise Emmett and Prof Oliver Sartor, as well as the SRC.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis", which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic (TCT) that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA are unregistered products. The safety and efficacy of 67Cu-SAR-bisPSMA and 64Cu-SAR-bisPSMA have not been assessed by health authorities such as the US FDA or the Therapeutic Goods Administration (TGA). There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death in men worldwide[5]. Prostate cancer is the second-leading causes of cancer death in American men. The American Cancer Institute estimates in 2025 there will be about 313,780 new cases of prostate cancer in the US and around 35,770 deaths from the disease[6].
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious diseases. The Company is a leader in innovative radiopharmaceuticals, developing TCTs based on its SAR Technology Platform for the treatment of cancers.
www.claritypharmaceuticals.com
For more information, please contact:
Clarity Pharmaceuticals
Dr Alan Taylor
Lisa Sadetskaya
Executive Chairperson
Director, Corporate Communications
ataylor@claritypharm.com
lisa@claritypharm.com
References
This announcement has been authorised for release by the Executive Chairperson.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

SECuRE trial to continue with no modifications to protocol following Safety Review Committee meeting















