WINNIPEG, Manitoba (AP) — Tomas Hertl scored on the power play at 4:47 of overtime and the Vegas Golden Knights beat Winnipeg 4-3 on Tuesday night, handing the Jets their 10th straight loss.
Reilly Smith had a goal and an assist, Mitch Marner also had a power-play goal, and Brett Howden also scored for the Golden Knights, who snapped a five-game skid (0-3-2). Carter Hart had 17 saves.
Click to Gallery
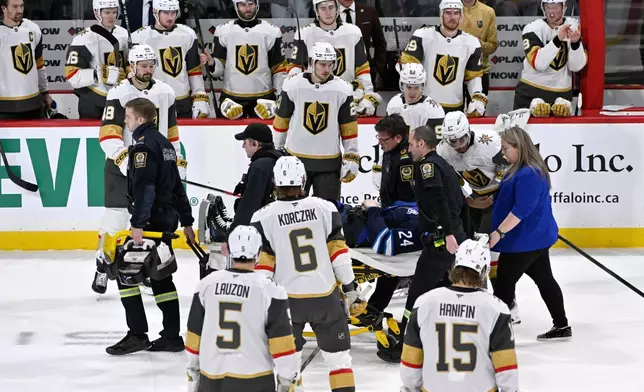
Winnipeg Jets' Haydn Fleury (24) is helped off the ice after being injured during the first period of an NHL hockey game against the Vegas Golden Knights in Winnipeg, Manitoba, Tuesday Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)
Vegas Golden Knights' Zach Whitecloud (2) passes the puck past Winnipeg Jets' Jonathan Toews (19) during the second period of an NHL hockey game in Winnipeg, Manitoba, Tuesday, Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)
Vegas Golden Knights' Mark Stone (61) and Winnipeg Jets goaltender Connor Hellebuyck (37) attempt to control a loose puck behind the net during the third period of their NHL hockey game in Winnipeg, Tuesday Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)
Vegas Golden Knights' Tomas Hertl (48) celebrates his game-winning goal in overtime against Winnipeg Jets goaltender Connor Hellebuyck (37) with Mark Stone (61) during their NHL hockey game in Winnipeg, Tuesday Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)
Vegas Golden Knights' Tomas Hertl (48) celebrates his game-winning goal against the Winnipeg Jets with Mitch Marner (93) and Jack Eichel (9) in overtime of their NHL hockey game in Winnipeg, Tuesday Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)
Cole Perfetti, Luke Schenn and Kyle Connor scored for Winnipeg, and Gabriel Vilardi had two assists. Connor Hellebuyck had 27 saves as the Jets fell to 0-6-4 during their losing streak.
With Winnipeg's Dylan Samberg off for tripping late in the extra period, Mitch Marner fired the puck and it bounced off Hertl and in as he was battling in front of Hellebuyck.
Perfetti beat Hart with a backhand 5:16 into the game to snap a 16-game goal drought. He skated by the boards and banged the glass in celebration.
The crowd was silenced after Jets defenseman Haydn Fleury was taken off the ice on a stretcher with just under seven minutes remaining in the first. Fleury was shoved by Vegas forward Keegan Kolesar near the bottom of the circle while clearing the puck and slid hard back-first into the end boards..
Schenn, playing in his 1,100th career game, fired a ploint shot through traffic and past Hart with 7:56 remaining in the first to push the Jets' lead to 2-0.
Stone got the Jets on the board with 51 seconds remaining in the second as he flipped a loose puck into the net 12 seconds into a man advantage for his 13th goal of the season. It extended his career-best goal streak to five games.
Howden took a pass across the front of the net from Noah Hanifin and tied the game 2-2 at 8:13 of the third. It gave him a goal in three straight games.
Connor gave the Jets the lead back with 5:04 remaining, but Smith scored 59 seconds later to tie it 3-3.
Golden Knights: Host Columbus on Thursday night.
Jets: Host Edmonton on Thursday night.
AP NHL: https://apnews.com/hub/nhl
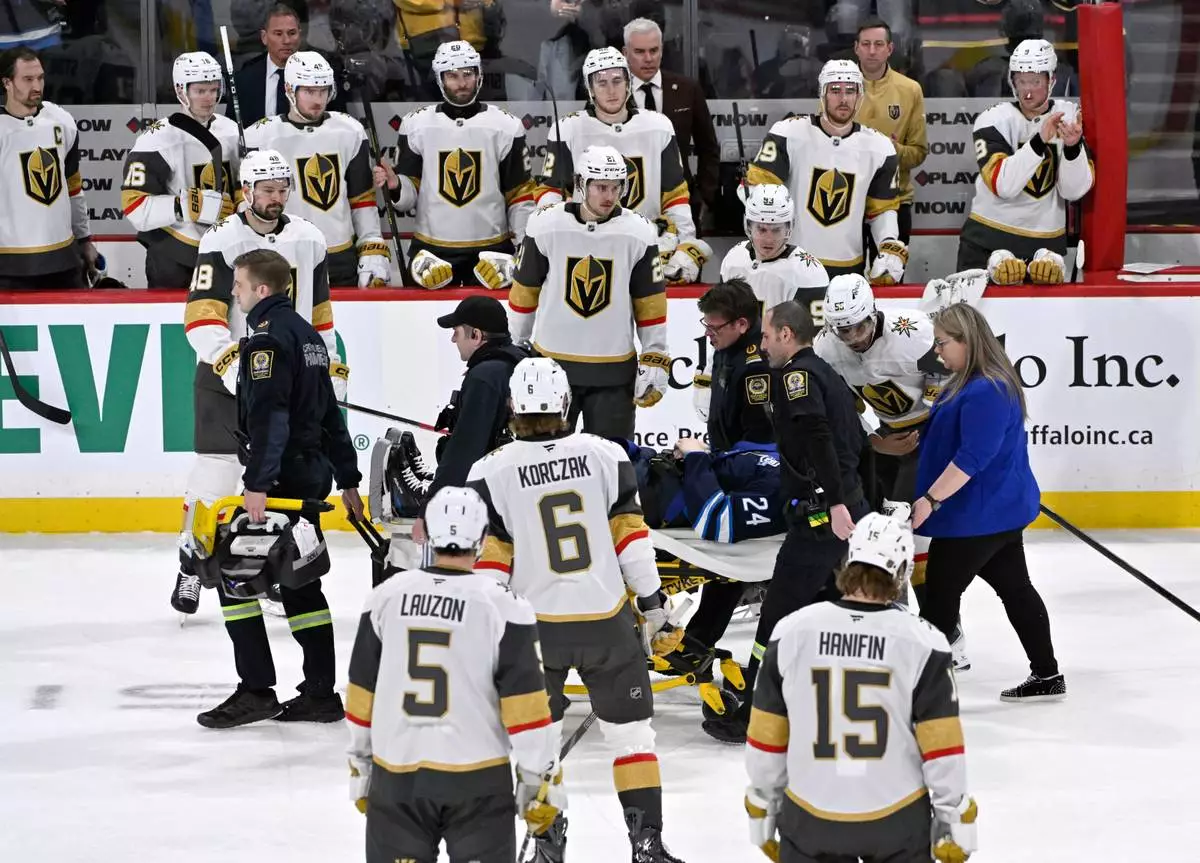
Winnipeg Jets' Haydn Fleury (24) is helped off the ice after being injured during the first period of an NHL hockey game against the Vegas Golden Knights in Winnipeg, Manitoba, Tuesday Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)

Vegas Golden Knights' Zach Whitecloud (2) passes the puck past Winnipeg Jets' Jonathan Toews (19) during the second period of an NHL hockey game in Winnipeg, Manitoba, Tuesday, Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)

Vegas Golden Knights' Mark Stone (61) and Winnipeg Jets goaltender Connor Hellebuyck (37) attempt to control a loose puck behind the net during the third period of their NHL hockey game in Winnipeg, Tuesday Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)

Vegas Golden Knights' Tomas Hertl (48) celebrates his game-winning goal in overtime against Winnipeg Jets goaltender Connor Hellebuyck (37) with Mark Stone (61) during their NHL hockey game in Winnipeg, Tuesday Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)

Vegas Golden Knights' Tomas Hertl (48) celebrates his game-winning goal against the Winnipeg Jets with Mitch Marner (93) and Jack Eichel (9) in overtime of their NHL hockey game in Winnipeg, Tuesday Jan. 6, 2026. (Fred Greenslade/The Canadian Press via AP)
LILLE, France & PARIS--(BUSINESS WIRE)--Jan 8, 2026--
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20260107798042/en/
The U.S. Food and Drug Administration (FDA) approval marks another strategic step in 4MB’s global clinical deployment across Europe, Canada, and now the U.S., reinforcing the company’s role as a front-runner in the race to deliver the first disease-modifying osteoarthritis drug (DMOAD) to patients worldwide. Preclinical studies have shown that 4P004 can modulate multiple biological markers across the whole joint, supporting its potential as a first-in-class DMOAD capable of slowing structural impairment and improving joint function.
“FDA’s clearance of our IND represents a major validation of our program and enables the full execution of our clinical strategy across Europe, Canada, and the United States,” said Luc Boblet, Chief Executive Officer of 4Moving Biotech. “This milestone strengthens our position as one of the most advanced DMOAD developers globally and brings us closer to demonstrating the transformative potential of 4P004 for the millions of patients worldwide who currently have no disease-modifying options.”
The INFLAM MOTION trial is a 3-month, multicenter, randomized, double-blind, placebo-controlled Phase 2a study designed to enroll 129 patients suffering from knee osteoarthritis with synovitis. The trial includes:
This unique combination of clinical, imaging, and biomarker elements forms the foundation for future interactions with regulatory agencies on accelerated or conditional approval pathways, thereby strategically positioning 4P004 within the highest-priority segment of OA drug development.
“4P004, a GLP-1 receptor agonist administered intra-articularly, allows specific targeting of the diseased joint and joint tissues, aiming to relieve pain while also addressing the underlying disease process, thus offering an exciting novel approach for patients living with painful knee osteoarthritis. As the U.S. Coordinating Investigator, I am pleased to support the INFLAM MOTION study and look forward to evaluating this promising therapeutic approach for patients with knee osteoarthritis.”Thomas J. Schnitzer, MD, PhD,Professor of Medicine,Northwestern University
Professor Francis Berenbaum, MD, PhD, Chief Medical Officer of 4MovingBiotech, concluded: “The regulatory progress across three major regions underscores the scientific robustness of 4P004. INFLAM MOTION is designed to deliver clinically meaningful pain improvement while generating high-resolution structural and biological data to guide the next stage of development. We believe 4P004 has the potential to redefine how osteoarthritis is treated.”
4Moving Biotech will initiate patient enrollment in the United States in Q1 2026, following site activation and investigator onboarding.
About 4Moving Biotech
Founded in 2020 as a spin-off of 4P-Pharma, 4Moving Biotech is a clinical-stage biotechnology company developing disease-modifying drugs for osteoarthritis, one of the world’s most burdensome chronic diseases, affecting more than 600 million people and lacking approved therapies that alter disease course. Headquartered on the Pasteur Institute campus in Lille, 4MB aims to deliver safe, sustainable therapeutic solutions for patients with high unmet medical needs.
Website: www.4movingbiotech.com
LinkedIn: https://fr.linkedin.com/company/4moving-biotech
X : https://x.com/4Moving_Biotech

4Moving Biotech (4MB), a clinical stage biotechnology company developing next-generation, disease-modifying therapies for osteoarthritis (OA), today announced that the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) application for 4P004, enabling the expansion of the Phase 2a INFLAM MOTION clinical trial into the United States (US).