Global Trade Observatory Annual Outlook: 94% expect 2026 growth to match or exceed 2025 despite tariffs, costs and policy uncertainty
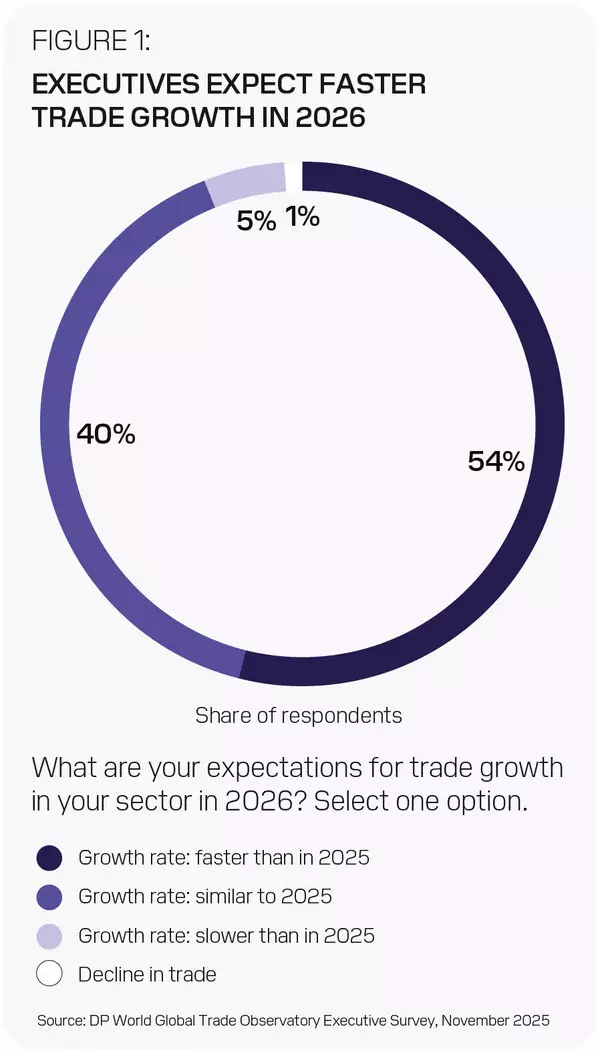
DAVOS, Switzerland, Jan. 20, 2026 /PRNewswire/ -- The global trade outlook looks fragile. Business confidence does not. That's the core finding of DP World's new Global Trade Observatory (GTO) Annual Outlook Report 2026, showing 94% of respondents expect 2026 trade growth to match or exceed the pace of 2025, despite rising frictions and volatility.
The findings are based on a survey of 3,500 senior supply chain and logistics executives across eight industries and 19 countries, conducted ahead of the World Economic Forum Annual Meeting in Davos.
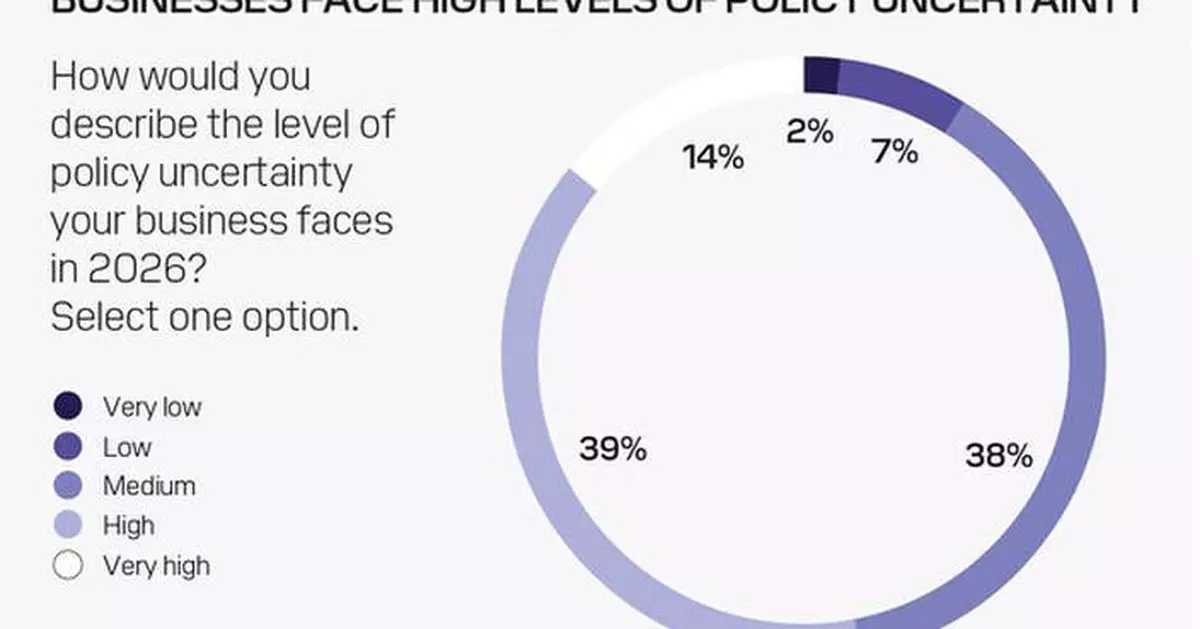
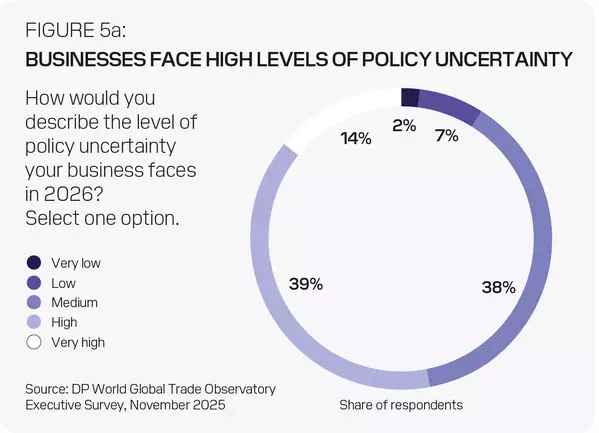
In total, 54% expect trade growth to be faster than 2025 and 40% expect it to be equal. This is despite 53% anticipating high or very high policy uncertainty, 90% expecting trade barriers to rise or remain unchanged. Only 25% expect a negative impact on their business, with 49% expecting no effect and 26% even seeing a positive impact.
This frontline sentiment contrasts with some macro projections, with the IMF forecasting trade growth (by volume) could slow to 2.3% in 2026, down from an estimated 3.6% in 2025.
Asked where trade growth potential is greatest in 2026, executives most frequently pointed to Europe (22%) and China (17%), followed by Asia Pacific (14%) and North America (13%).
Sultan Ahmed bin Sulayem, Group Chairman and CEO of DP World, said: "Global trade is becoming increasingly complex, not less so. Our role is clear: to keep trade moving by understanding where friction exists, anticipating where it may emerge next, and investing in the infrastructure, capabilities and partnerships that help our customers operate more efficiently and reliably."
The GTO Annual Outlook was developed with Geneva-based insights agency, Horizon Group. Margareta Drzeniek, Managing Partner, Horizon Group, said: "What we're seeing is confidence with contingency plans. Executives are embedding resilience into strategy by diversifying suppliers, reassessing routes and adding options, because volatility is now the baseline. Those best positioned will be the ones who can turn those resilience plans into measurable performance."
What companies are doing differently in 2026
The survey indicates companies are responding to volatility by actively redesigning supply chains and trade routes. This includes:
- Resilience as strategy: Supplier diversification (51%), higher inventories (44%), and friend-shoring (36%) are cited among the most common strategic shifts planned for 2026.
- Route agility increases: 26% intend to use new routes, while 23% are evaluating them. Decisions are driven by cost savings (38%), improved connectivity/inland infrastructure (36%), and faster customs procedures/clearance times (35%).
- Border friction remains a choke point: 60% cite customs clearance as a leading cause of delays and disruption. Executives also prioritise investment in warehousing and logistics hubs (39%), road networks (36%), and border/customs processing infrastructure (36%).
The DP World Global Trade Observatory (GTO) is a data- and insights-led platform designed to provide decision-makers with actionable intelligence on the forces reshaping global trade, grounded in research including a proprietary survey of 3,500 supply chain and logistics executives across eight industries and 19 countries. Research was conducted in November 2025 with Geneva-based insights agency Horizon Group.
For media enquiries, please contact:
Adal Mirza
Group Vice President
Adal.mirza@dpworld.com
+971 50 628 7856
Hakam Kherallah
Group Senior Manager
Hakam.Kherallah@dpworld.com
+971 50 552 2610
Follow DP World on:
X (Twitter): https://twitter.com/DP_World
LinkedIn: https://www.linkedin.com/company/dp-world
About DP World
DP World is reshaping the future of global trade to improve lives everywhere. Operating across six continents with a team of over 125,000 employees, we combine global infrastructure and local expertise to deliver seamless supply chain solutions. From Ports and Terminals to Marine Services, Logistics and Technology, we leverage innovation to create better ways to trade, minimizing disruptions from the factory floor to the customer's door.
WE MAKE TRADE FLOW
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

DP WORLD SURVEY: TRADE LEADERS UPBEAT ON 2026 DESPITE RISING BARRIERS
DP WORLD SURVEY: TRADE LEADERS UPBEAT ON 2026 DESPITE RISING BARRIERS
DP WORLD SURVEY: TRADE LEADERS UPBEAT ON 2026 DESPITE RISING BARRIERS