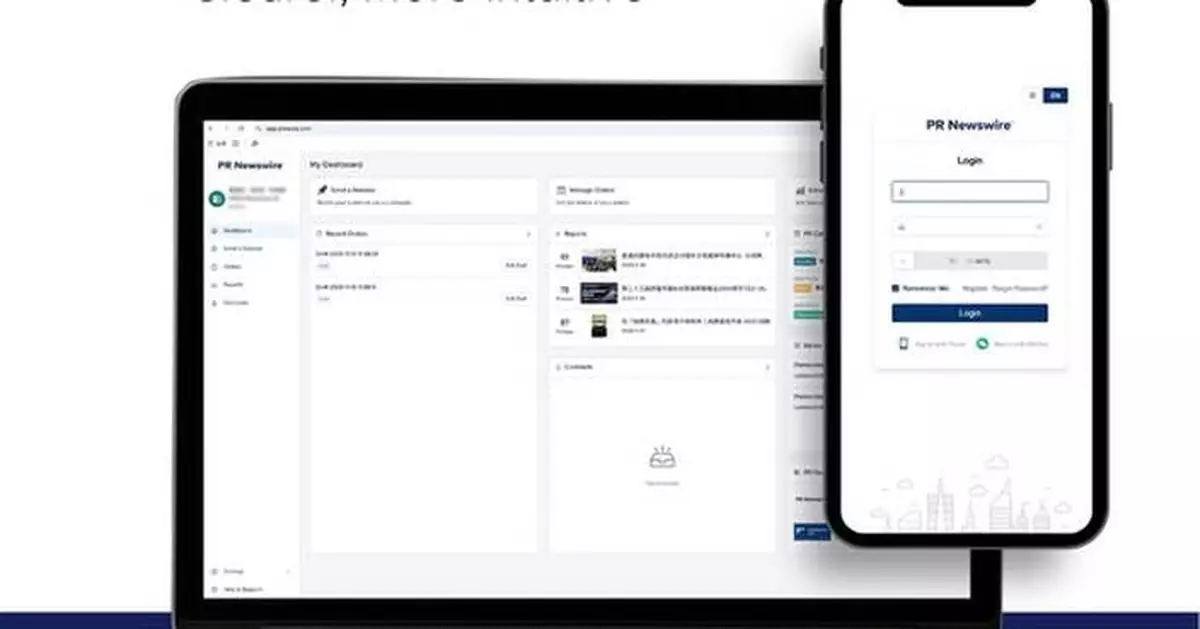
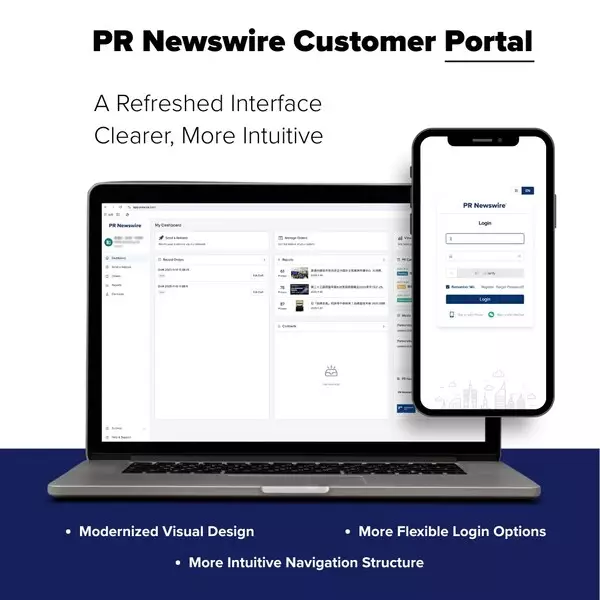
BEIJING, Jan. 29, 2026 /PRNewswire/ -- PR Newswire has completed a major upgrade to its Customer Portal in Asia Pacific, featuring a refreshed interface and an enhanced performance report. The portal now offers a more intuitive, efficient experience for clients distributing press releases and managing orders.
Key Enhancements Include:
- Streamlined design delivering a seamless, consistent and mobile-responsive experience.
- Clearer navigation and simplified distribution and management workflows.
- Faster, more flexible login options, including mobile number and WeChat.
These enhancements are designed to better support customers by simplifying day‑to‑day workflows and making it easier to access, understand, and act on the campaign performance data.
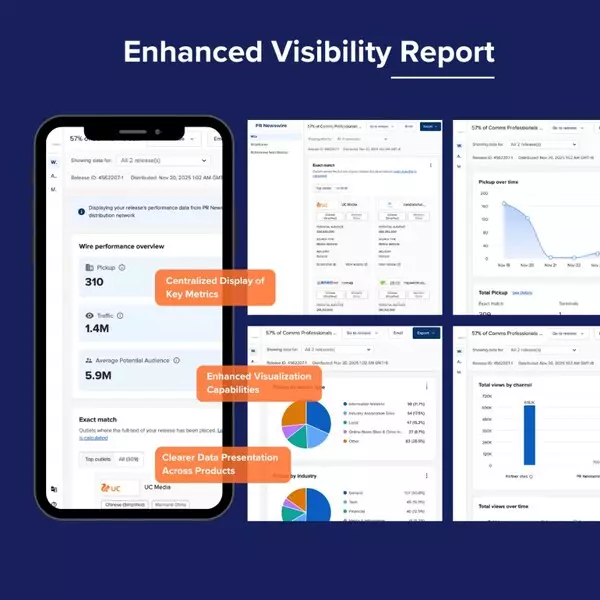
The enhanced Visibility Report has been completely redesigned to deliver major improvements in visualization, data structure, and usability, giving clients clearer insights into press release performance and communication impact.
Key Enhancements Include:
- Standardized Data Presentation: Performance data for News Releases, Amplification, and Multichannel News Releases (MNR) is now presented in a consistent format, making comparison across all release types easier.
- Centralized Key Metrics: Core metrics such as pickup, traffic, and potential audience are consolidated into a single dashboard to improve readability and save time.
- Enhanced Visualization: Modernized charts and layouts provide clearer visibility into performance across all channels.
These improvements enable PR and communications teams to gain insights faster, make data‑driven decisions, and deliver a better overall experience for their audiences.
These new features will further support PR Newswire's clients in their day‑to‑day workflows, offering a smoother experience and more actionable insights to guide communication strategies. Clients will be informed individually in the coming weeks about the migration to the new platform and are encouraged to contact the Client Relations team at apaccs@cision.com with any questions.
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **
PR Newswire Customer Portal Upgrade Introduces New Interface and Enhanced Visibility Report
PR Newswire Customer Portal Upgrade Introduces New Interface and Enhanced Visibility Report
|
- Global phase 1/2 clinical trial initiated for GBP511, a vaccine targeting the Sarbecovirus Family
- Next-generation vaccine leveraging SKYCovione's recombinant protein-based platform and nanoparticle technology
- SK bioscience will continue to strengthen a Mid- to Long-Term global vaccine strategy centered on universal vaccines for future pandemic preparedness
INCHEON, South Korea, Jan. 29, 2026 /PRNewswire/ -- SK bioscience announced the initiation of a global Phase 1/2 clinical trial in Australia for GBP511, a vaccine candidate targeting the sarbecovirus subgenus.
Sarbecoviruses are a higher-level coronavirus group that include SARS-CoV-2, encompassing not only currently circulating variants but also potential future novel coronaviruses related SARS-like coronaviruses found in animals that could potentially spill over to humans." Or even just, "related SARS-like coronaviruses found in animals.
SK bioscience aims to develop a universal vaccine capable of inducing broad immune responses across the entire virus family and is establishing a platform applicable to multiple related viruses and variants.
The international Phase 1/2 trial of GBP511 will be conducted in approximately 368 adults aged 18 years and older in Australia. In Phase 1, participants will receive two doses administered 28 days apart, across low-, medium-, and high-dose cohorts, with or without an adjuvant. Safety, tolerability, and immunogenicity will be evaluated, including comparisons with Comirnaty, a comparator vaccine. Based on Phase 1 results, the optimal dose and regimen will be selected, after which Phase 2 will assess immunogenicity and safety in adult and elderly populations, again comparing the investigational vaccine with Comirnaty, a comparator vaccine. In addition, the study will evaluate cross-reactive immune responses across the sarbecovirus family, including SARS-CoV-2, to assess the vaccine's potential as a universal coronavirus vaccine.
GBP511 incorporates the core technology of of SKYCovione, which in 2022 became the only domestically developed COVID-19 vaccine in Korea to achieve commercialization. Building on SKYCovione's computer-designed antigen platform, GBP511 combines SK bioscience's recombinant protein technology with self-assembling nanoparticle design technology developed by the Institute for Protein Design (IPD) at the University of Washington School of Medicine. SKYCovione has previously demonstrated robust immunogenicity and a favorable safety profile through global clinical trials. SKYCovione demonstrated the induction of robust protective neutralizing antibody levels and a favorable safety profile through global clinical trials, which was the basis of the vaccine's approval by several regulatory authorities.
Globally, while multiple companies and research institutions have initiated efforts to develop universal coronavirus vaccines, most programs remain at an early research stage.
SK bioscience's entry into global Phase 1/2 clinical development is the first such vaccine to enter clinical trials, demonstrating a comparatively rapid pace of development.
The Coalition for Epidemic Preparedness Innovations (CEPI) has designated the SARS-coronavirus family as a "prototype pathogen," noting the high likelihood that future pandemics may repeatedly arise from the same viral family. CEPI has consistently emphasized the limitations of variant- or pathogen-specific approaches and highlighted the need for universal coronavirus vaccines capable of providing broad protection regardless of the emergence of novel viruses. CEPI is supporting the development of broadly protective coronavirus vaccines, which could potentially provide broad protection against the Betacoronavirus family, which includes SARS and MERS.
Major international journals, including Nature Reviews Immunology and The Lancet Microbe, have likewise reported that universal sarbecovirus vaccines capable of delivering both broad cross-neutralizing activity and durable immune protection could become a cornerstone of future pandemic preparedness strategies.
Against this backdrop, the COVID-19 vaccine market is expected to transition from short-term outbreak-driven demand to sustained mid- to long-term growth. According to global market research firm Coherent Market Insights, the global COVID-19 vaccine market is estimated at approximately USD 50.6 billion (KRW 70 trillion) in 2025 and is projected to grow at a compound annual growth rate of 7.4% from 2025 to 2032, reaching approximately USD 83.4 billion (KRW 117 trillion) by 2032. Next-generation technologies, such as universal vaccines, are expected to generate new sources of market demand.
Dr Richard Hatchett, CEO of CEPI said, "COVID 19 exposed the devastating price of confronting a deadly new virus without a vaccine. Broadly protective coronavirus vaccines have the power to change that story. While we cannot predict when or where the next coronavirus threat will emerge, we can prepare. By investing now in all in one vaccines, CEPI and its partners are strengthening global defences—and South Korea is playing a pivotal role in that effort. The progression of SK bioscience's broadly protective coronavirus vaccine into Phase 1/2 trials marks a major step forward, showcasing South Korea's leadership in cutting-edge vaccine innovation and bringing us closer to a safer future for all."
Jaeyong Ahn, CEO of SK bioscience said "Developing a universal sarbecovirus vaccine is a critical challenge that must be addressed to prepare for the next pandemic." "With the initiation of the GBP511 clinical trial, we will accelerate the development of universal vaccines and leverage our proactive infectious disease preparedness capabilities to emerge as a global leader in the vaccine market."
SK bioscience continues to advance a range of infectious disease pipelines, including a 21-valent pneumococcal conjugate vaccine and an avian influenza vaccine. The company plans to progressively expand its vaccine portfolio to strengthen mid- to long-term preparedness for future infectious disease threats.
About SK bioscience
SK bioscience is an innovative vaccine and biotech company, committed to vaccine development and manufacturing to enable more equitable access to vaccines around the world. Leveraging strengths on cutting-edge technologies, SK bioscience has been dedicated to promoting human health from prevention to cure across the globe. With the cooperation of domestic and international governments, regulatory agencies, healthcare providers, doctors, and medical experts, all of the SK colleagues are passionately committed to providing high-quality vaccines to those who need them and better public healthcare solutions.
SK bioscience Communications Team
Changhyun Jin (jin99@sk.com)
Tae-Gyun Kim (taegyunkim@sk.com)
Moonchel Kim (mc_kim@sk.com)
- Global phase 1/2 clinical trial initiated for GBP511, a vaccine targeting the Sarbecovirus Family
- Next-generation vaccine leveraging SKYCovione's recombinant protein-based platform and nanoparticle technology
- SK bioscience will continue to strengthen a Mid- to Long-Term global vaccine strategy centered on universal vaccines for future pandemic preparedness
INCHEON, South Korea, Jan. 29, 2026 /PRNewswire/ -- SK bioscience announced the initiation of a global Phase 1/2 clinical trial in Australia for GBP511, a vaccine candidate targeting the sarbecovirus subgenus.
Sarbecoviruses are a higher-level coronavirus group that include SARS-CoV-2, encompassing not only currently circulating variants but also potential future novel coronaviruses related SARS-like coronaviruses found in animals that could potentially spill over to humans." Or even just, "related SARS-like coronaviruses found in animals.
SK bioscience aims to develop a universal vaccine capable of inducing broad immune responses across the entire virus family and is establishing a platform applicable to multiple related viruses and variants.
The international Phase 1/2 trial of GBP511 will be conducted in approximately 368 adults aged 18 years and older in Australia. In Phase 1, participants will receive two doses administered 28 days apart, across low-, medium-, and high-dose cohorts, with or without an adjuvant. Safety, tolerability, and immunogenicity will be evaluated, including comparisons with Comirnaty, a comparator vaccine. Based on Phase 1 results, the optimal dose and regimen will be selected, after which Phase 2 will assess immunogenicity and safety in adult and elderly populations, again comparing the investigational vaccine with Comirnaty, a comparator vaccine. In addition, the study will evaluate cross-reactive immune responses across the sarbecovirus family, including SARS-CoV-2, to assess the vaccine's potential as a universal coronavirus vaccine.
GBP511 incorporates the core technology of of SKYCovione, which in 2022 became the only domestically developed COVID-19 vaccine in Korea to achieve commercialization. Building on SKYCovione's computer-designed antigen platform, GBP511 combines SK bioscience's recombinant protein technology with self-assembling nanoparticle design technology developed by the Institute for Protein Design (IPD) at the University of Washington School of Medicine. SKYCovione has previously demonstrated robust immunogenicity and a favorable safety profile through global clinical trials. SKYCovione demonstrated the induction of robust protective neutralizing antibody levels and a favorable safety profile through global clinical trials, which was the basis of the vaccine's approval by several regulatory authorities.
Globally, while multiple companies and research institutions have initiated efforts to develop universal coronavirus vaccines, most programs remain at an early research stage.
SK bioscience's entry into global Phase 1/2 clinical development is the first such vaccine to enter clinical trials, demonstrating a comparatively rapid pace of development.
The Coalition for Epidemic Preparedness Innovations (CEPI) has designated the SARS-coronavirus family as a "prototype pathogen," noting the high likelihood that future pandemics may repeatedly arise from the same viral family. CEPI has consistently emphasized the limitations of variant- or pathogen-specific approaches and highlighted the need for universal coronavirus vaccines capable of providing broad protection regardless of the emergence of novel viruses. CEPI is supporting the development of broadly protective coronavirus vaccines, which could potentially provide broad protection against the Betacoronavirus family, which includes SARS and MERS.
Major international journals, including Nature Reviews Immunology and The Lancet Microbe, have likewise reported that universal sarbecovirus vaccines capable of delivering both broad cross-neutralizing activity and durable immune protection could become a cornerstone of future pandemic preparedness strategies.
Against this backdrop, the COVID-19 vaccine market is expected to transition from short-term outbreak-driven demand to sustained mid- to long-term growth. According to global market research firm Coherent Market Insights, the global COVID-19 vaccine market is estimated at approximately USD 50.6 billion (KRW 70 trillion) in 2025 and is projected to grow at a compound annual growth rate of 7.4% from 2025 to 2032, reaching approximately USD 83.4 billion (KRW 117 trillion) by 2032. Next-generation technologies, such as universal vaccines, are expected to generate new sources of market demand.
Dr Richard Hatchett, CEO of CEPI said, "COVID 19 exposed the devastating price of confronting a deadly new virus without a vaccine. Broadly protective coronavirus vaccines have the power to change that story. While we cannot predict when or where the next coronavirus threat will emerge, we can prepare. By investing now in all in one vaccines, CEPI and its partners are strengthening global defences—and South Korea is playing a pivotal role in that effort. The progression of SK bioscience's broadly protective coronavirus vaccine into Phase 1/2 trials marks a major step forward, showcasing South Korea's leadership in cutting-edge vaccine innovation and bringing us closer to a safer future for all."
Jaeyong Ahn, CEO of SK bioscience said "Developing a universal sarbecovirus vaccine is a critical challenge that must be addressed to prepare for the next pandemic." "With the initiation of the GBP511 clinical trial, we will accelerate the development of universal vaccines and leverage our proactive infectious disease preparedness capabilities to emerge as a global leader in the vaccine market."
SK bioscience continues to advance a range of infectious disease pipelines, including a 21-valent pneumococcal conjugate vaccine and an avian influenza vaccine. The company plans to progressively expand its vaccine portfolio to strengthen mid- to long-term preparedness for future infectious disease threats.
About SK bioscience
SK bioscience is an innovative vaccine and biotech company, committed to vaccine development and manufacturing to enable more equitable access to vaccines around the world. Leveraging strengths on cutting-edge technologies, SK bioscience has been dedicated to promoting human health from prevention to cure across the globe. With the cooperation of domestic and international governments, regulatory agencies, healthcare providers, doctors, and medical experts, all of the SK colleagues are passionately committed to providing high-quality vaccines to those who need them and better public healthcare solutions.
SK bioscience Communications Team
Changhyun Jin (jin99@sk.com)
Tae-Gyun Kim (taegyunkim@sk.com)
Moonchel Kim (mc_kim@sk.com)
** The press release content is from PR Newswire. Bastille Post is not involved in its creation. **

SK bioscience Initiates International Phase 1/2 Clinical Trial of Universal Vaccine Candidate Targeting Sarbecovirus Family