GALWAY, Ireland--(BUSINESS WIRE)--Mar 13, 2025--
Perfuze, a leading medical device company dedicated to advancing stroke treatment, today announced that it has received FDA 510(k) clearance for its Zipline™ Access Catheters. This regulatory milestone strengthens Perfuze’s growing portfolio of innovative neurovascular devices, empowering physicians to treat acute ischemic strokes with greater speed, ease, and precision. In addition, Perfuze has secured €22 million in follow-on funding in a round led by existing investors including Earlybird, EQT Life Sciences, Seroba and SV Health.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250313532644/en/
Transforming Stroke Care with Zipline ™
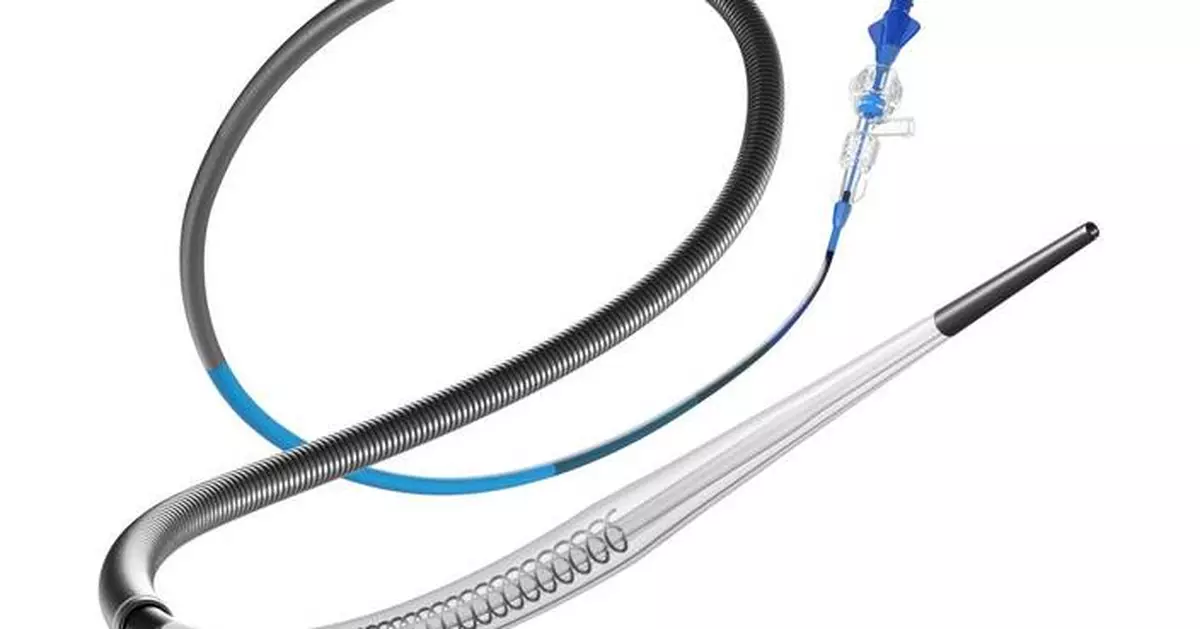
Every minute counts in stroke care. As a stroke progresses, human brain tissue is quickly and permanently lost, making urgent therapeutic intervention critical. The Zipline™ Access Catheters are engineered to enhance the trackability and delivery of large (070) and superbore (088) aspiration catheters, simplifying neurointerventional procedures and enabling faster, more efficient stroke treatment. By providing increased support and navigational ease, Zipline™ aims to optimize clot removal efficiency, ultimately improving procedural success rates and patient outcomes. The FDA clearance marks a significant step forward in Perfuze’s mission to simplify and improve stroke treatment and outcomes through technological innovation.
“The FDA clearance of our Zipline™ Access Catheter is a testament to Perfuze’s commitment to developing best-in-class stroke solutions,” said Wayne Allen, CEO of Perfuze. “This regulatory approval strengthens our growing presence in the U.S. market and supports our vision of delivering novel, effective, and easy-to-use technologies that can make a real difference in stroke care.” Dr. Jay Dolia, Assistant Professor, Neurology, Emory University School of Medicine stated: “The Zipline catheters represent an innovative technology that I believe will simplify stroke intervention, reduce costs and accelerate reperfusion. In my initial experience, they have enabled rapid clot access and aspiration, even in complex anatomy."
Fueling Growth: €22 Million Investment to Drive Commercial Rollout
Perfuze has successfully closed a €22 million funding round, led by existing investors. This investment will support the Limited Market Release of the Zipline™ and Millipede™ catheters while advancing ongoing clinical and R&D initiatives to further enhance stroke treatment capabilities.
Perfuze has initiated a Limited Market Release in the U.S. in prestigious US Comprehensive Stroke Centres. The company’s growing portfolio is designed to provide physicians with highly effective tools to simplify stroke treatment and improve patient outcomes. “The continued support from our investors underscores the confidence in Perfuze’s technology and vision,” stated Perfuze Chairperson, Hooman Hakami. “With this funding, we are well-positioned to initiate our U.S. Limited Market Release and drive adoption in select centers of our transformative stroke treatment solutions.”
For more information about Perfuze and its innovative stroke treatment solutions, visit www.perfuze.com.
About Perfuze
Perfuze is a medical device company headquartered in Galway, Ireland, dedicated to developing next-generation catheter-based aspiration technology for the treatment of acute ischemic stroke. The company’s proprietary platform is designed to maximize clot removal efficiency, enhance procedural simplicity, and improve clinical outcomes.


Zipline Access Catheter (with coil) delivering the Millipede 088 catheter (Photo: Business Wire)